Chemical Kinetics Unit 11 Chemical Kinetics Chemical equations


![Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p](https://slidetodoc.com/presentation_image_h2/94c8f29381163a37f42c039d2cd378c2/image-9.jpg)

![Deriving Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate goes Deriving Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate goes](https://slidetodoc.com/presentation_image_h2/94c8f29381163a37f42c039d2cd378c2/image-15.jpg)



- Slides: 34

Chemical Kinetics Unit 11

Chemical Kinetics Chemical equations do not give us information on how fast a reaction goes from reactants to products. n KINETICS: the study of reaction rates and their relation to the way the reaction proceeds, i. e. its mechanism n We can use thermodynamics to tell if a reaction is product – or reactant – favored n Only kinetics will tell us how fast the reaction happens! n

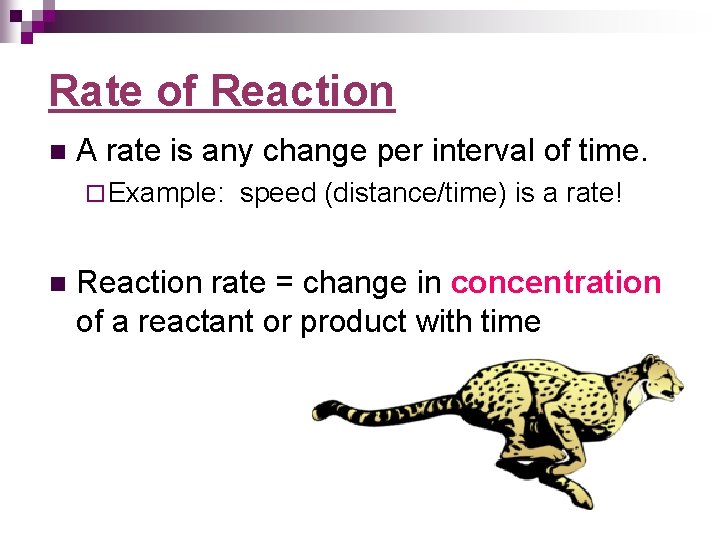
Rate of Reaction n A rate is any change per interval of time. ¨ Example: n speed (distance/time) is a rate! Reaction rate = change in concentration of a reactant or product with time

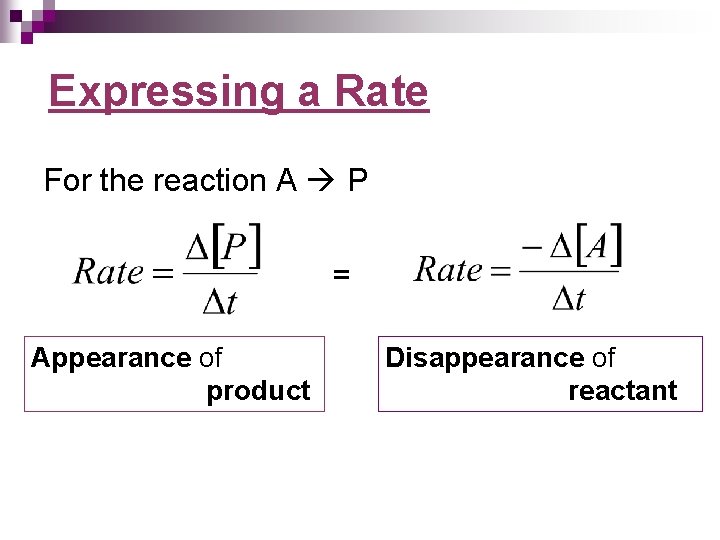
Expressing a Rate For the reaction A P = Appearance of product Disappearance of reactant

Reaction Conditions & Rates Collision Theory of Reactants n Reactions occur when molecules collide to exchange or rearrange atoms n Effective collisions occur when molecules have correct energy and orientation

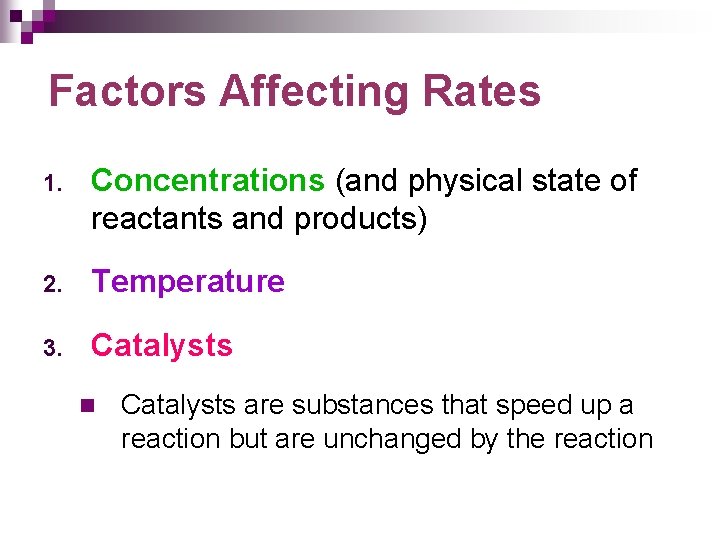
Factors Affecting Rates 1. Concentrations (and physical state of reactants and products) 2. Temperature 3. Catalysts n Catalysts are substances that speed up a reaction but are unchanged by the reaction

Effect of Concentration on Reaction Rate To propose a reaction mechanism, we study the reaction rate and its concentration dependence.

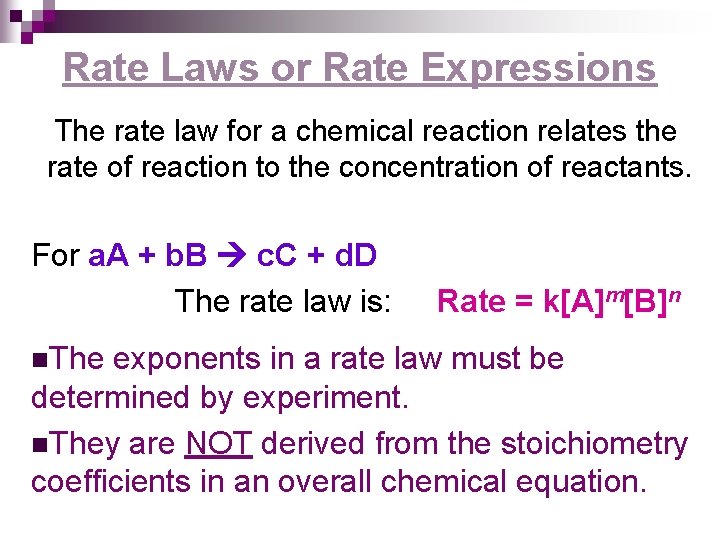
Rate Laws or Rate Expressions The rate law for a chemical reaction relates the rate of reaction to the concentration of reactants. For a. A + b. B c. C + d. D The rate law is: n. The Rate = k[A]m[B]n exponents in a rate law must be determined by experiment. n. They are NOT derived from the stoichiometry coefficients in an overall chemical equation.
![Rate Laws Orders of Reactions Rate Law for a reaction Rate kAmBnCp Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p](https://slidetodoc.com/presentation_image_h2/94c8f29381163a37f42c039d2cd378c2/image-9.jpg)
Rate Laws & Orders of Reactions Rate Law for a reaction: Rate = k[A]m[B]n[C]p The exponents m, n, and p ¨ Are the reaction order ¨ Can be 0, 1, 2, or fractions (may be other whole numbers in fictional examples) ¨ Must be determined by experiment Overall Order = sum of m, n, and p

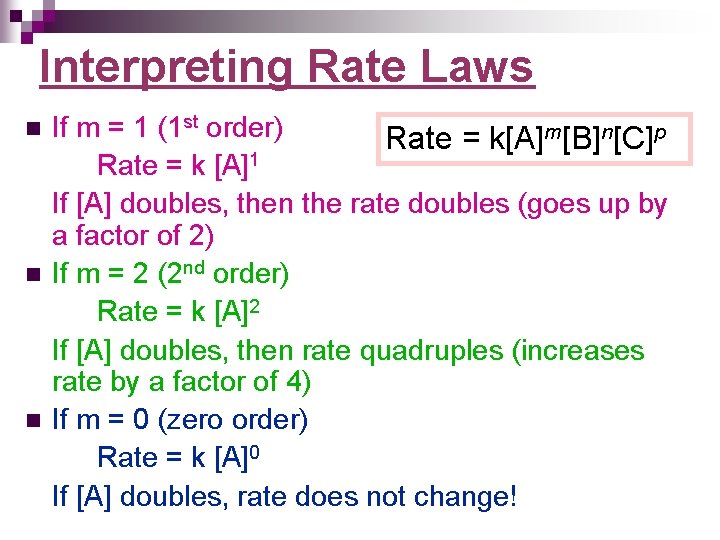
Interpreting Rate Laws n n n If m = 1 (1 st order) Rate = k[A]m[B]n[C]p Rate = k [A]1 If [A] doubles, then the rate doubles (goes up by a factor of 2) If m = 2 (2 nd order) Rate = k [A]2 If [A] doubles, then rate quadruples (increases rate by a factor of 4) If m = 0 (zero order) Rate = k [A]0 If [A] doubles, rate does not change!

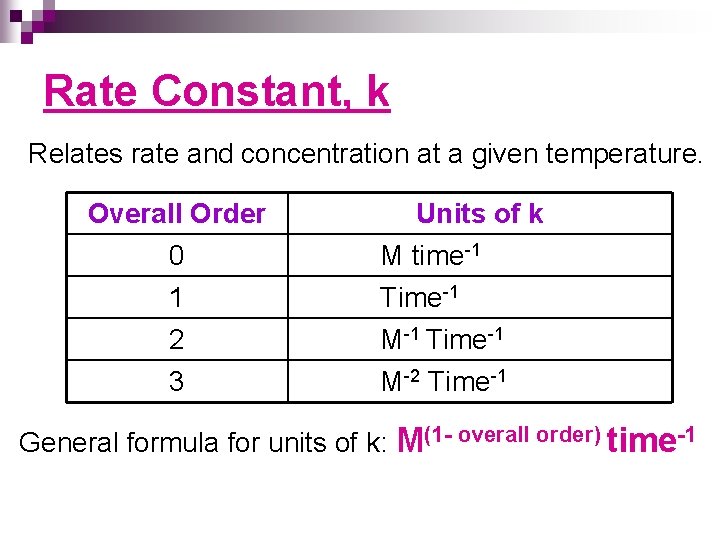
Rate Constant, k Relates rate and concentration at a given temperature. Overall Order 0 1 2 3 Units of k M time-1 Time-1 M-2 Time-1 General formula for units of k: M(1 - overall order) time-1

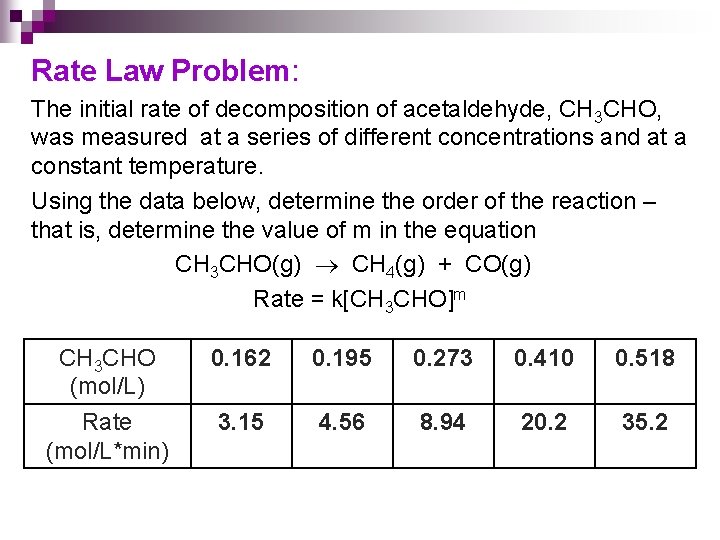
Rate Law Problem: The initial rate of decomposition of acetaldehyde, CH 3 CHO, was measured at a series of different concentrations and at a constant temperature. Using the data below, determine the order of the reaction – that is, determine the value of m in the equation CH 3 CHO(g) CH 4(g) + CO(g) Rate = k[CH 3 CHO]m CH 3 CHO (mol/L) 0. 162 0. 195 0. 273 0. 410 0. 518 Rate (mol/L*min) 3. 15 4. 56 8. 94 20. 2 35. 2

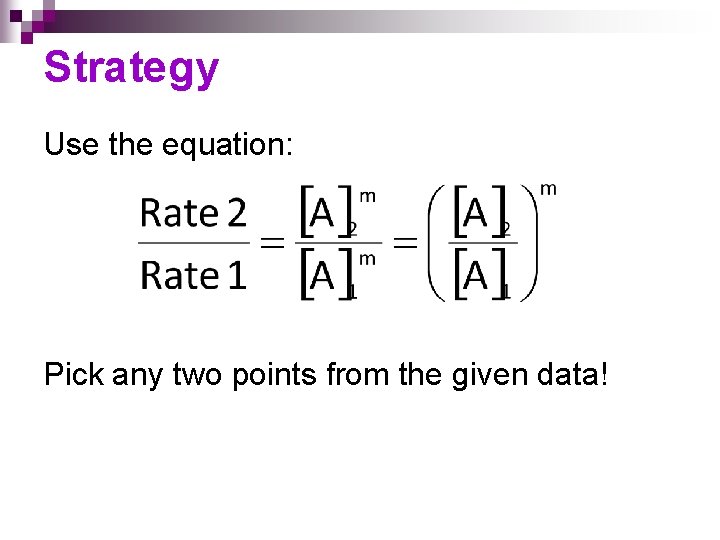
Strategy Use the equation: Pick any two points from the given data!

![Deriving Rate Laws Rate of rxn kCH 3 CHO2 Here the rate goes Deriving Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate goes](https://slidetodoc.com/presentation_image_h2/94c8f29381163a37f42c039d2cd378c2/image-15.jpg)
Deriving Rate Laws Rate of rxn = k[CH 3 CHO]2 Here the rate goes up by FOUR when the initial concentration doubles. Therefore, we say this reaction is SECOND order overall.

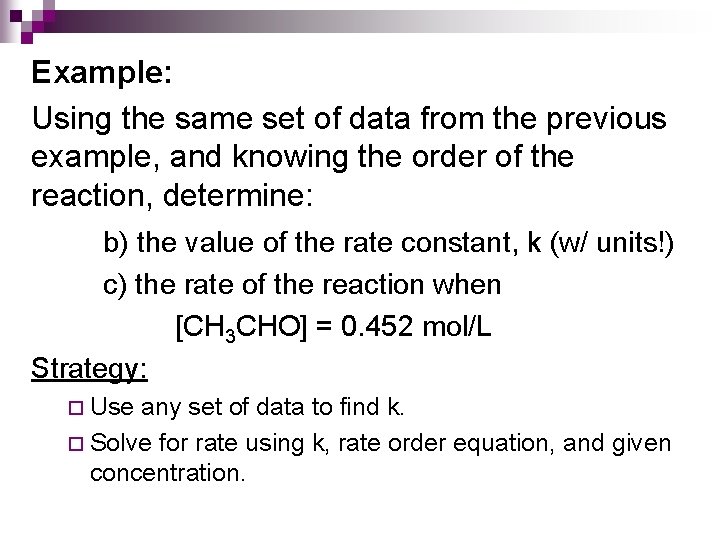
Example: Using the same set of data from the previous example, and knowing the order of the reaction, determine: b) the value of the rate constant, k (w/ units!) c) the rate of the reaction when [CH 3 CHO] = 0. 452 mol/L Strategy: ¨ Use any set of data to find k. ¨ Solve for rate using k, rate order equation, and given concentration.


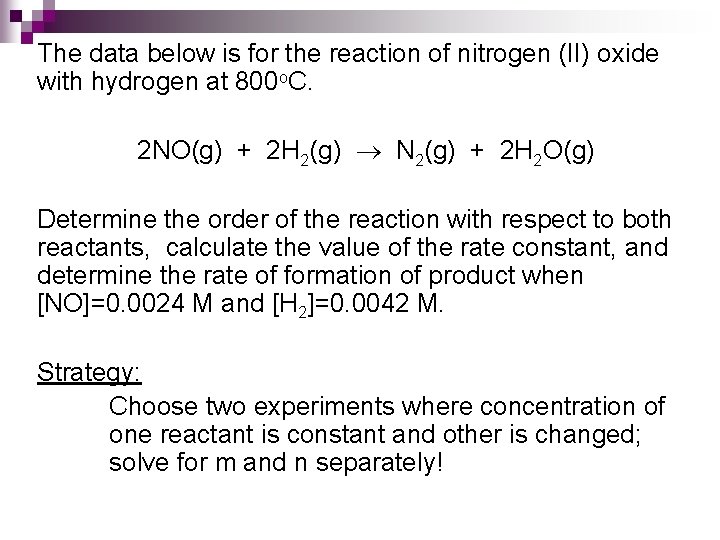
The data below is for the reaction of nitrogen (II) oxide with hydrogen at 800 o. C. 2 NO(g) + 2 H 2(g) N 2(g) + 2 H 2 O(g) Determine the order of the reaction with respect to both reactants, calculate the value of the rate constant, and determine the rate of formation of product when [NO]=0. 0024 M and [H 2]=0. 0042 M. Strategy: Choose two experiments where concentration of one reactant is constant and other is changed; solve for m and n separately!


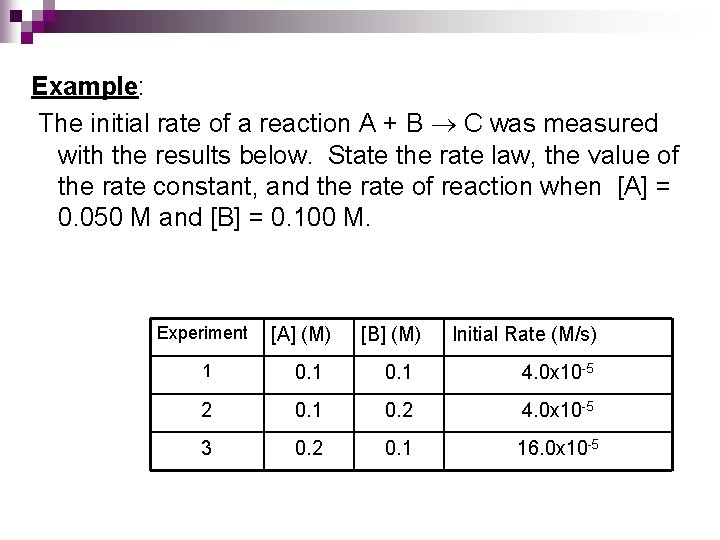
Example: The initial rate of a reaction A + B C was measured with the results below. State the rate law, the value of the rate constant, and the rate of reaction when [A] = 0. 050 M and [B] = 0. 100 M. Experiment [A] (M) [B] (M) Initial Rate (M/s) 1 0. 1 4. 0 x 10 -5 2 0. 1 0. 2 4. 0 x 10 -5 3 0. 2 0. 1 16. 0 x 10 -5


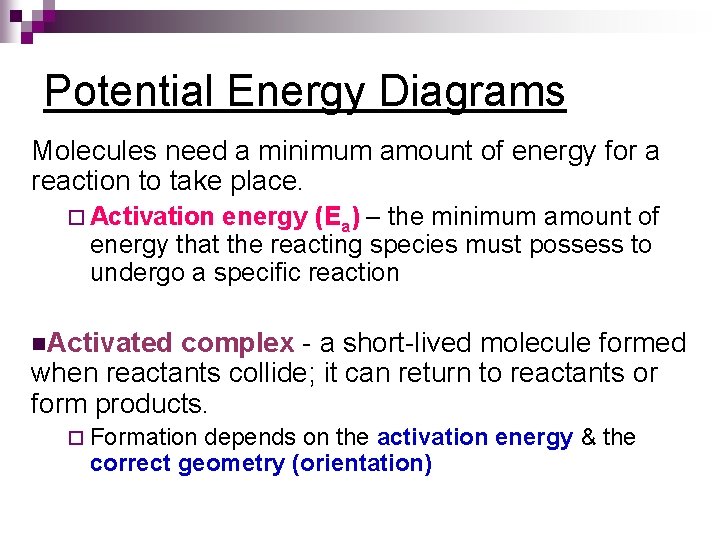
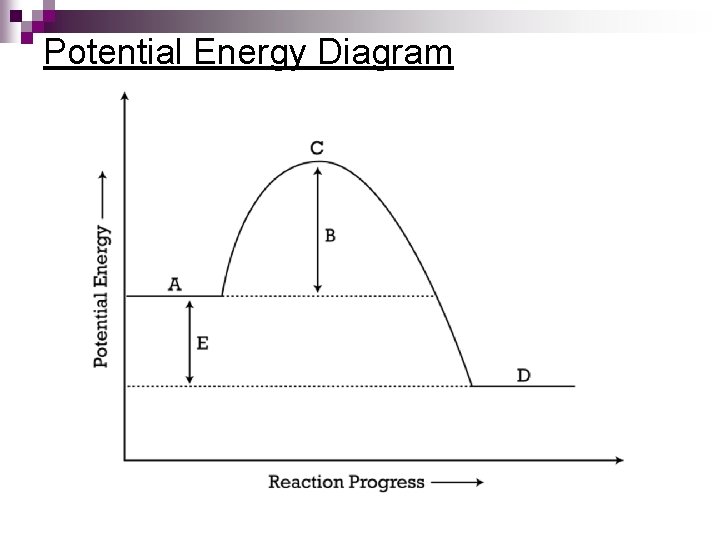
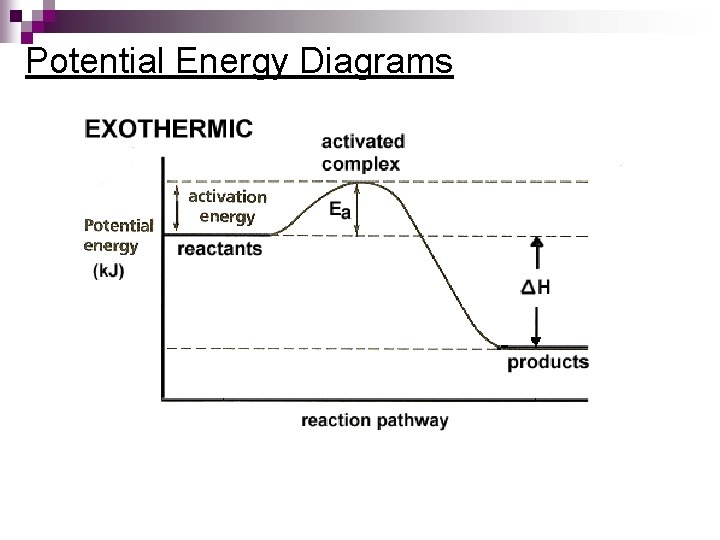
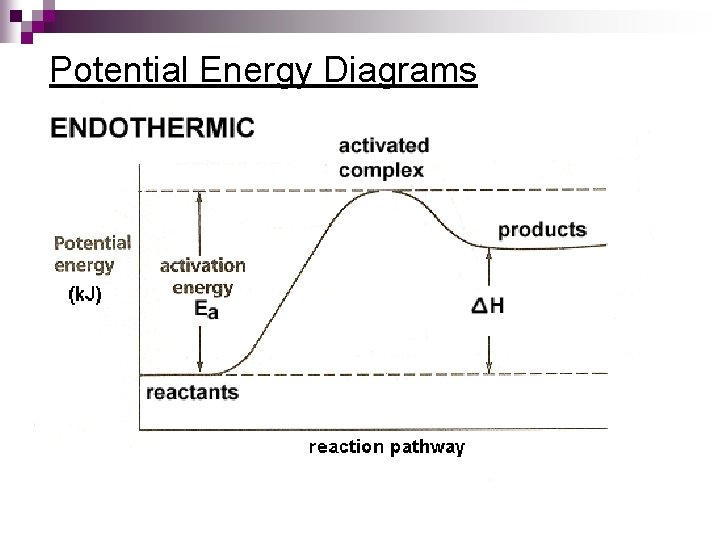
Potential Energy Diagrams Molecules need a minimum amount of energy for a reaction to take place. ¨ Activation energy (Ea) – the minimum amount of energy that the reacting species must possess to undergo a specific reaction n. Activated complex - a short-lived molecule formed when reactants collide; it can return to reactants or form products. ¨ Formation depends on the activation energy & the correct geometry (orientation)

Potential Energy Diagram

Potential Energy Diagrams

Potential Energy Diagrams

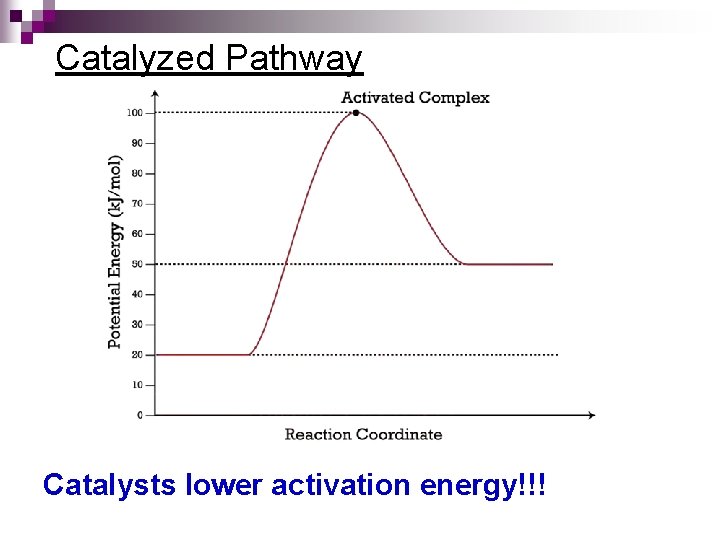
Catalyzed Pathway Catalysts lower activation energy!!!

Reaction Mechanisms Mechanism – how reactants are converted to products at the molecular level Most reactions DO NOT occur in a single step! They occur as a series of elementary steps (a single step in a reaction).

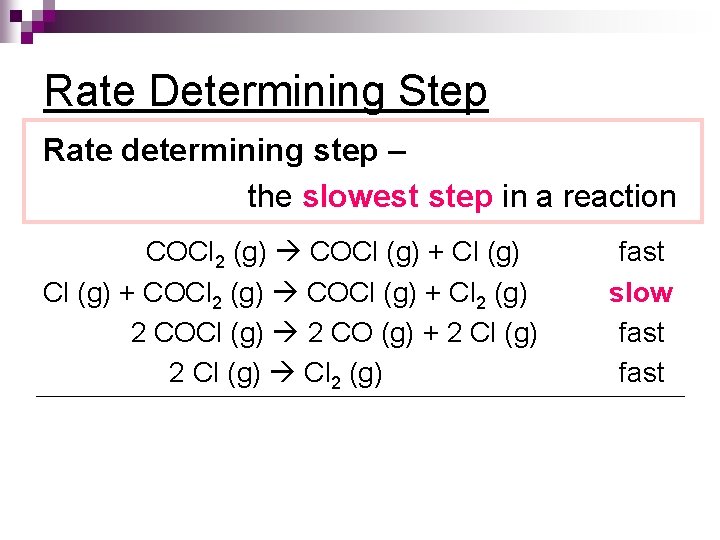
Rate Determining Step Rate determining step – the slowest step in a reaction COCl 2 (g) COCl (g) + COCl 2 (g) COCl (g) + Cl 2 (g) 2 COCl (g) 2 CO (g) + 2 Cl (g) Cl 2 (g) fast slow fast

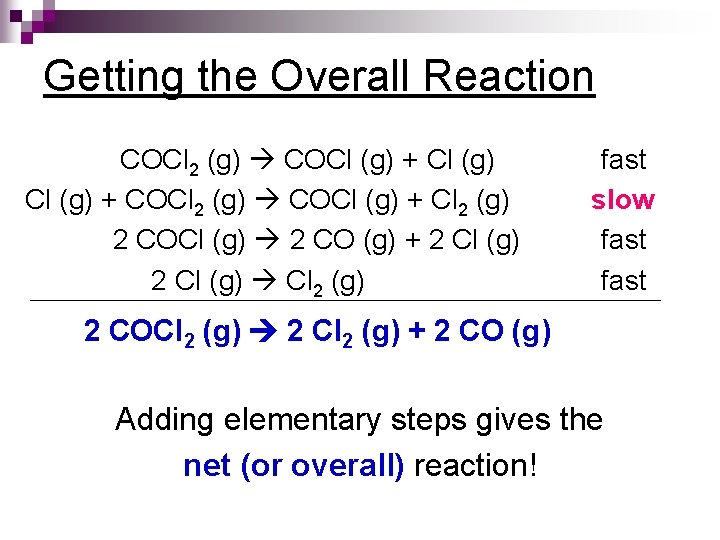
Getting the Overall Reaction COCl 2 (g) COCl (g) + COCl 2 (g) COCl (g) + Cl 2 (g) 2 COCl (g) 2 CO (g) + 2 Cl (g) Cl 2 (g) fast slow fast 2 COCl 2 (g) 2 Cl 2 (g) + 2 CO (g) Adding elementary steps gives the net (or overall) reaction!

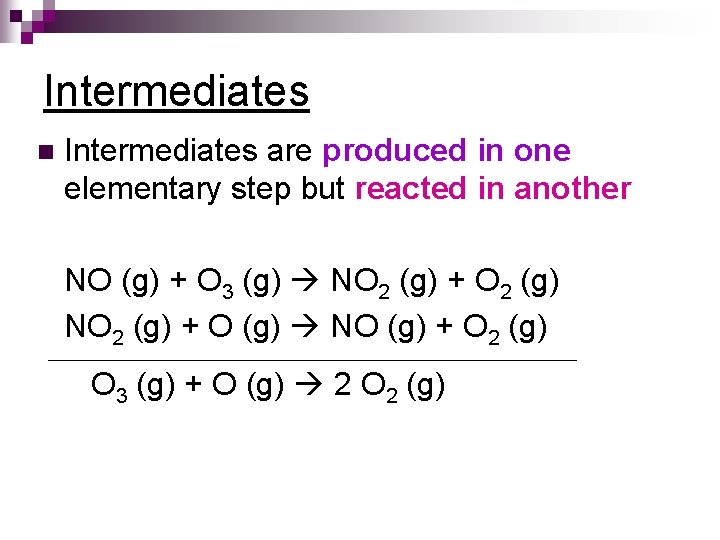
Intermediates n Intermediates are produced in one elementary step but reacted in another NO (g) + O 3 (g) NO 2 (g) + O 2 (g) NO 2 (g) + O (g) NO (g) + O 2 (g) O 3 (g) + O (g) 2 O 2 (g)

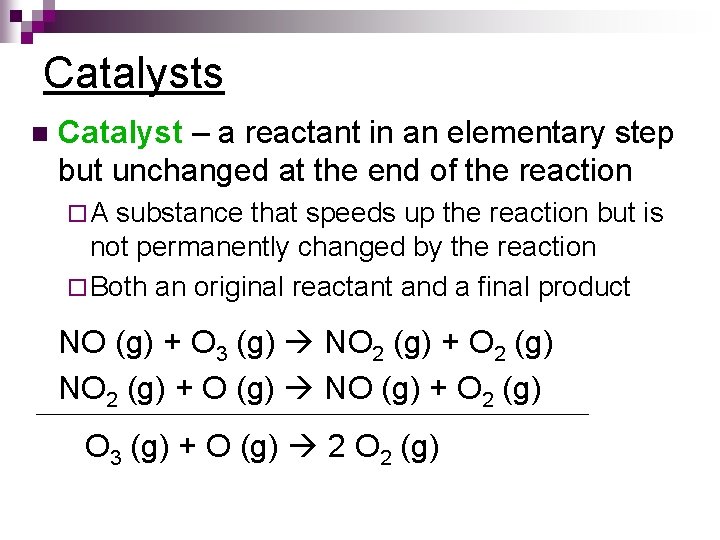
Catalysts n Catalyst – a reactant in an elementary step but unchanged at the end of the reaction ¨A substance that speeds up the reaction but is not permanently changed by the reaction ¨ Both an original reactant and a final product NO (g) + O 3 (g) NO 2 (g) + O 2 (g) NO 2 (g) + O (g) NO (g) + O 2 (g) O 3 (g) + O (g) 2 O 2 (g)

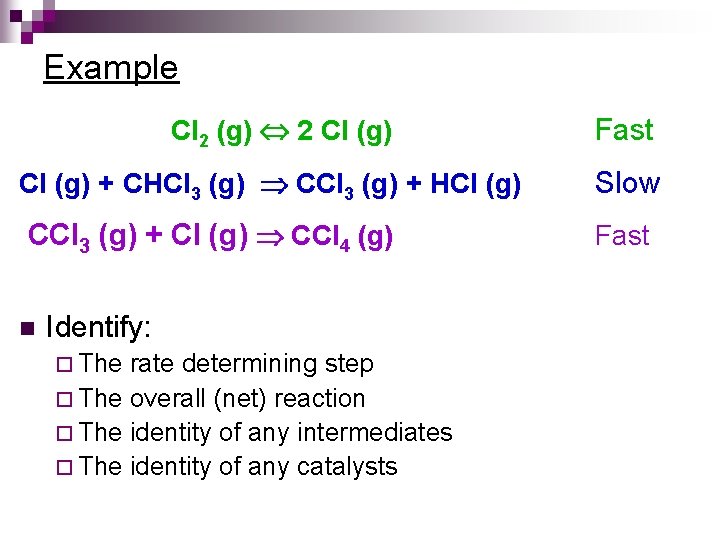
Example Cl 2 (g) 2 Cl (g) Fast Cl (g) + CHCl 3 (g) CCl 3 (g) + HCl (g) Slow CCl 3 (g) + Cl (g) CCl 4 (g) Fast n Identify: ¨ The rate determining step ¨ The overall (net) reaction ¨ The identity of any intermediates ¨ The identity of any catalysts

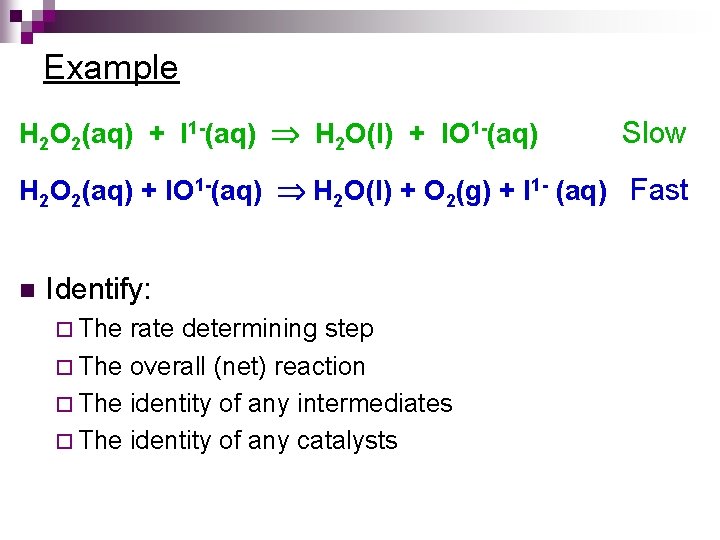
Example H 2 O 2(aq) + I 1 -(aq) H 2 O(l) + IO 1 -(aq) Slow H 2 O 2(aq) + IO 1 -(aq) H 2 O(l) + O 2(g) + I 1 - (aq) Fast n Identify: ¨ The rate determining step ¨ The overall (net) reaction ¨ The identity of any intermediates ¨ The identity of any catalysts

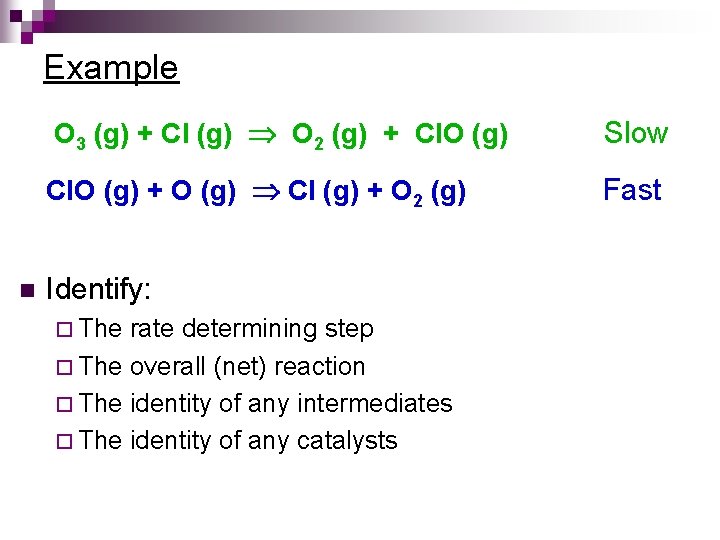
Example n O 3 (g) + Cl (g) O 2 (g) + Cl. O (g) Slow Cl. O (g) + O (g) Cl (g) + O 2 (g) Fast Identify: ¨ The rate determining step ¨ The overall (net) reaction ¨ The identity of any intermediates ¨ The identity of any catalysts