CHEMICAL KINETICS STUDY OF THE RATES OF CHEMICAL












- Slides: 23

CHEMICAL KINETICS – STUDY OF THE RATES OF CHEMICAL REACTIONS SOME REACTIONS WE WOULD LIKE TO SPEED UP MAKING FIREWORKS/EXPLOSIONS MAKING GASOLINE/PETROLEUM PRODUCTS SOME REACTIONS WE WOULD LIKE TO SLOW DOWN FIRE !! RUSTING CORRISION HW 11 -1, p. 101, Part 1, #1

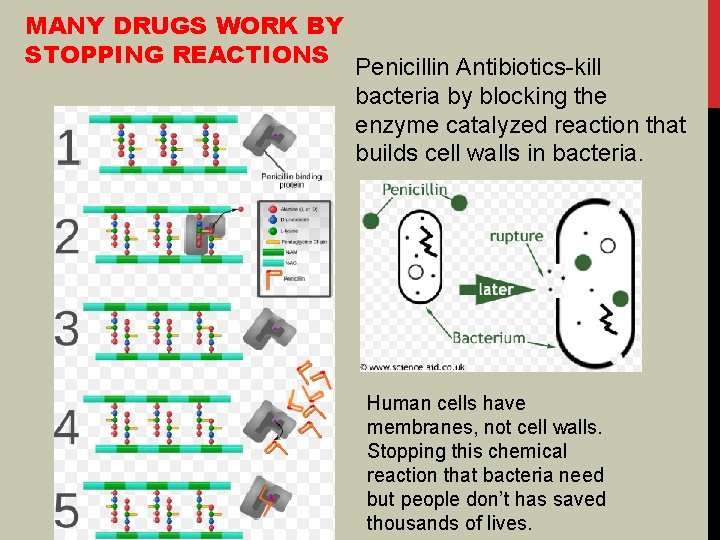
MANY DRUGS WORK BY STOPPING REACTIONS Penicillin Antibiotics-kill bacteria by blocking the enzyme catalyzed reaction that builds cell walls in bacteria. Human cells have membranes, not cell walls. Stopping this chemical reaction that bacteria need but people don’t has saved thousands of lives.

HW 11 -1 #2 – WAYS TO SPEED UP OR SLOW DOWN REACTIONS SPEED UP • STIRRING • INCREASE CONCENTRATION OF REACTANTS (or pressure of a gas system) • INCREASE SURFACE AREA • INCREASE TEMPERATURE • ADD A CATALYST SLOW DOWN • DECREASE CONC. • DECREASE SURFACE AREA • DECREASE TEMPERATURE • ADD INHIBITOR

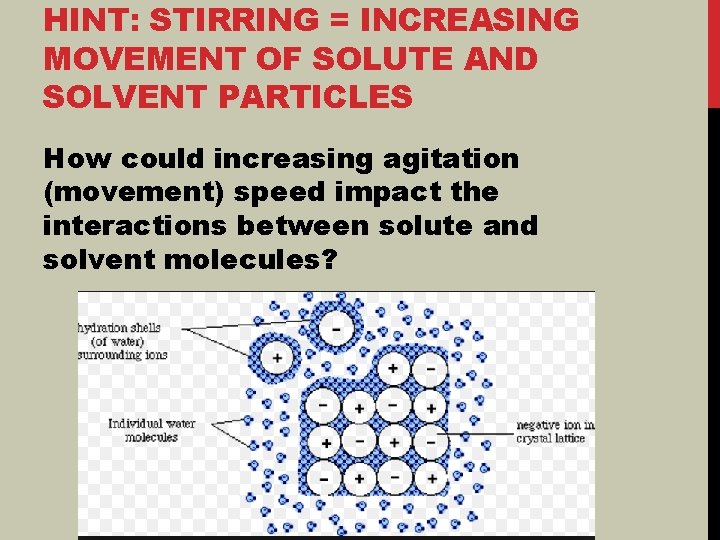
DEVELOPING AN ATOMIC LEVEL MODEL FOR REACTIONS WHY DOES STIRRING IMPACT REACTION RATE OR RATE OF DISSOLVING?

HINT: STIRRING = INCREASING MOVEMENT OF SOLUTE AND SOLVENT PARTICLES How could increasing agitation (movement) speed impact the interactions between solute and solvent molecules?

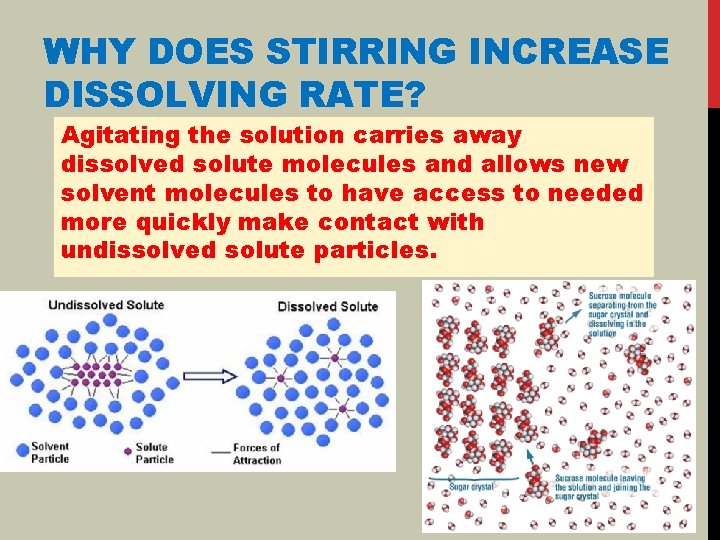
WHY DOES STIRRING INCREASE DISSOLVING RATE? Agitating the solution carries away dissolved solute molecules and allows new solvent molecules to have access to needed more quickly make contact with undissolved solute particles.

EFFECT OF CONCENTRATION ELEPHANT TOOTHPASTE RXN Link elephant toothpaste 4) In the first experiment with the green food coloring the hydrogen peroxide is a at a lower concentration. In the second experiment with the red food coloring the hydrogen peroxide is a a higher concentration. Which is faster? ANS: Higher concentration = Faster Reaction

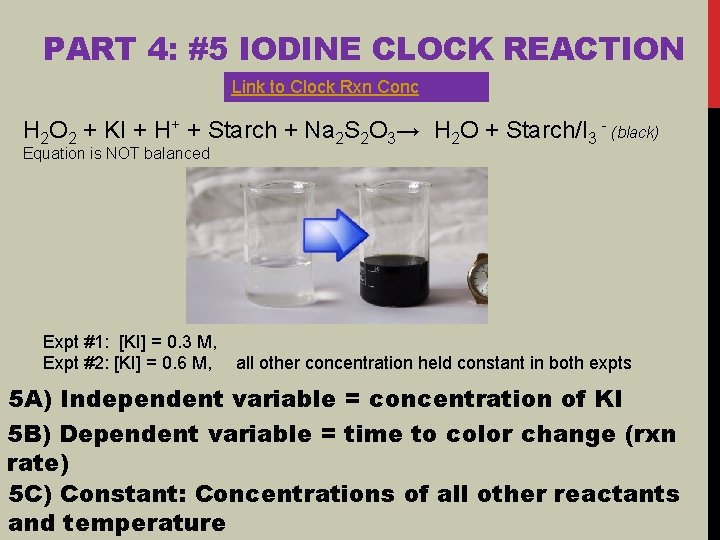
PART 4: #5 IODINE CLOCK REACTION Link to Clock Rxn Conc H 2 O 2 + KI + H+ + Starch + Na 2 S 2 O 3→ H 2 O + Starch/I 3 - (black) Equation is NOT balanced Expt #1: [KI] = 0. 3 M, Expt #2: [KI] = 0. 6 M, all other concentration held constant in both expts 5 A) Independent variable = concentration of KI 5 B) Dependent variable = time to color change (rxn rate) 5 C) Constant: Concentrations of all other reactants and temperature

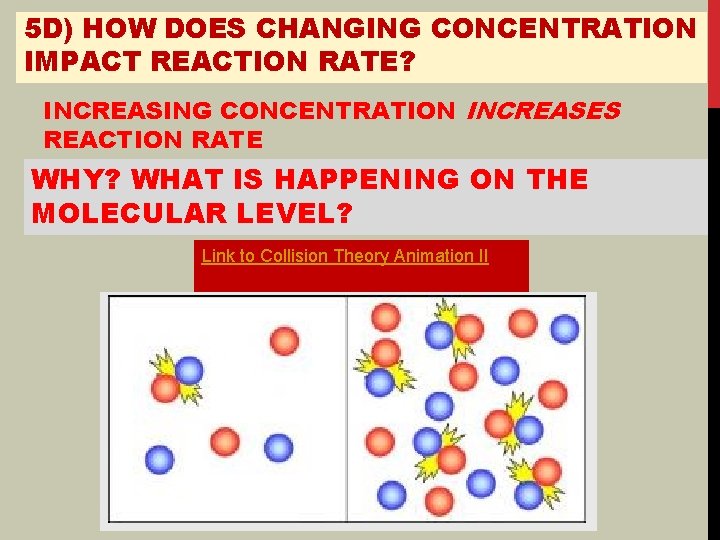
5 D) HOW DOES CHANGING CONCENTRATION IMPACT REACTION RATE? INCREASING CONCENTRATION INCREASES REACTION RATE WHY? WHAT IS HAPPENING ON THE MOLECULAR LEVEL? Link to Collision Theory Animation II

5 E) IN ORDER TO REACTANT MOLECULES MUST FIRST COLLIDE. INCREASING CONCENTRATION INCREASES # OF COLLISIONS ANSWER 7 F AND 7 G ON TOP OF p. 99 (Misnumbered, should be 5 f and 5 g)

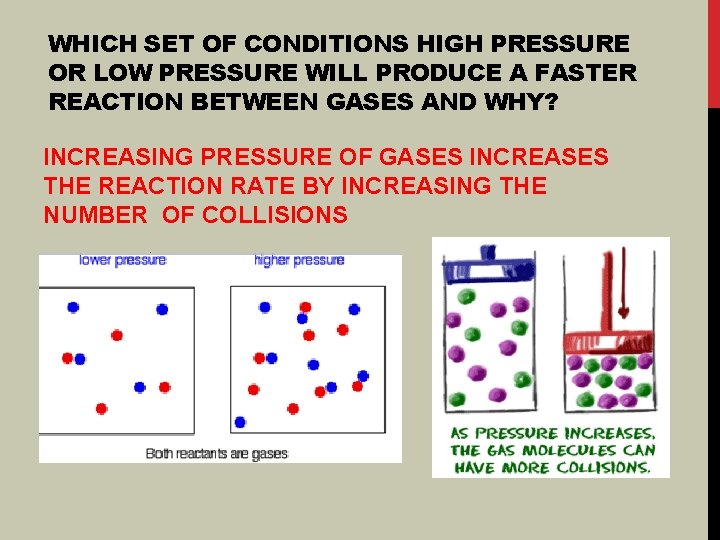
WHICH SET OF CONDITIONS HIGH PRESSURE OR LOW PRESSURE WILL PRODUCE A FASTER REACTION BETWEEN GASES AND WHY? INCREASING PRESSURE OF GASES INCREASES THE REACTION RATE BY INCREASING THE NUMBER OF COLLISIONS

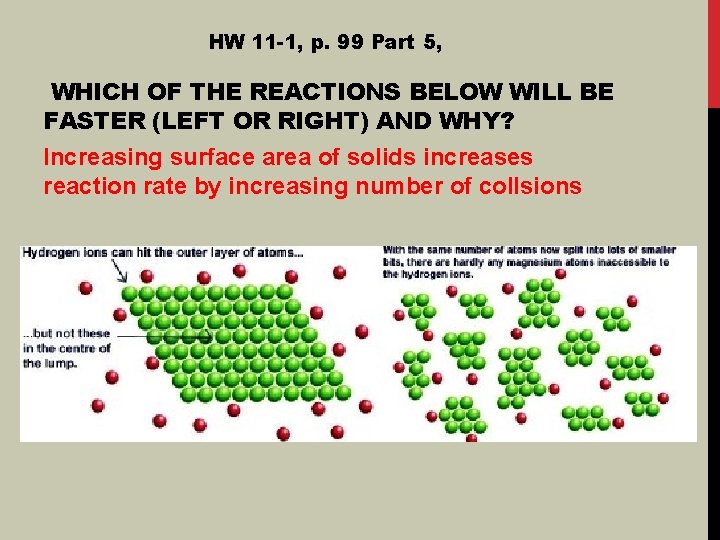
HW 11 -1, p. 99 Part 5, WHICH OF THE REACTIONS BELOW WILL BE FASTER (LEFT OR RIGHT) AND WHY? Increasing surface area of solids increases reaction rate by increasing number of collsions

WHICH HAS MORE SURFACE AREA, A STRIP OF IRON OR STEEL WOOL? WHICH REACTS MORE QUICKLY WITH OXYGEN? video link steel wool SA

WHICH BURNS MORE QUICKLY: A PILE OF CORNSTARCH OR BLOWN POWDER? Video corn flower SA

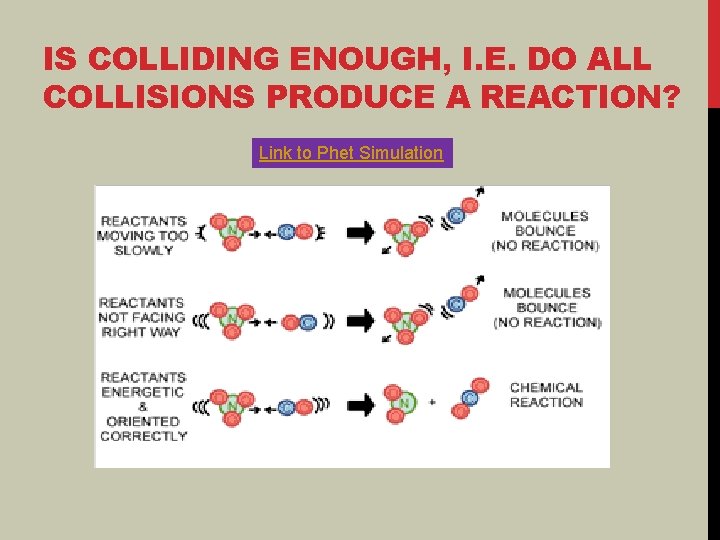
IS COLLIDING ENOUGH, I. E. DO ALL COLLISIONS PRODUCE A REACTION? Link to Phet Simulation

REACTION REQUIRES COLLISIONS IN THE CORRECT ORIENTATION The two specific atoms in the different molecules that are reacting have to collide in the correct orientation. HW 11 -1 Part 6 Link to animation

HW 11 -1, #11 BC AB (or BA)

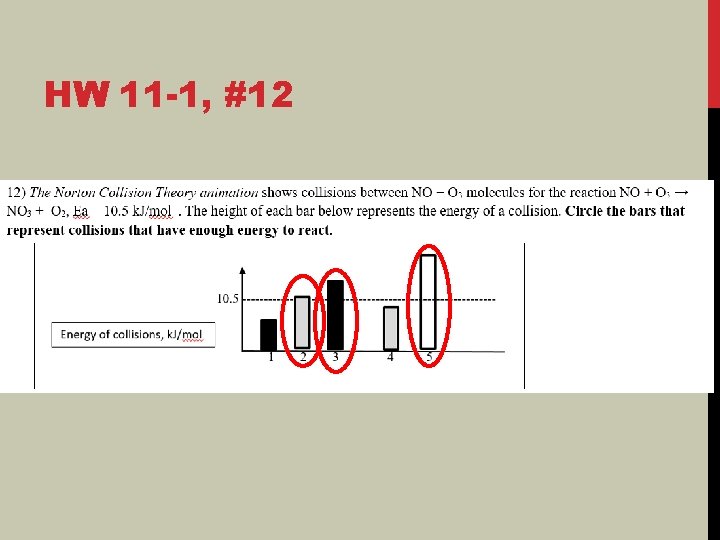
HW 11 -1: PART 12 ENERGY OF COLLISIONS Link to Phet Simulation 12 A) Does each correctly oriented collision produce a rxn? NO 12 B) In addition to the correct orientation, what other property of the collision is essential in order for the reaction to take place? ENERGY OF COLLISIONS 12 C) Activation Energy, Ea = ENERGY MINIMUM amount of necessary to initiate a chemical reaction.

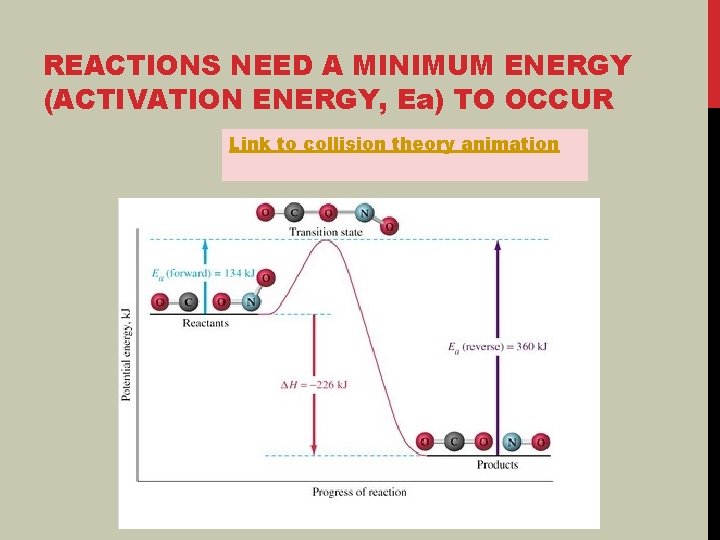
REACTIONS NEED A MINIMUM ENERGY (ACTIVATION ENERGY, Ea) TO OCCUR Link to collision theory animation

HW 11 -1, #12

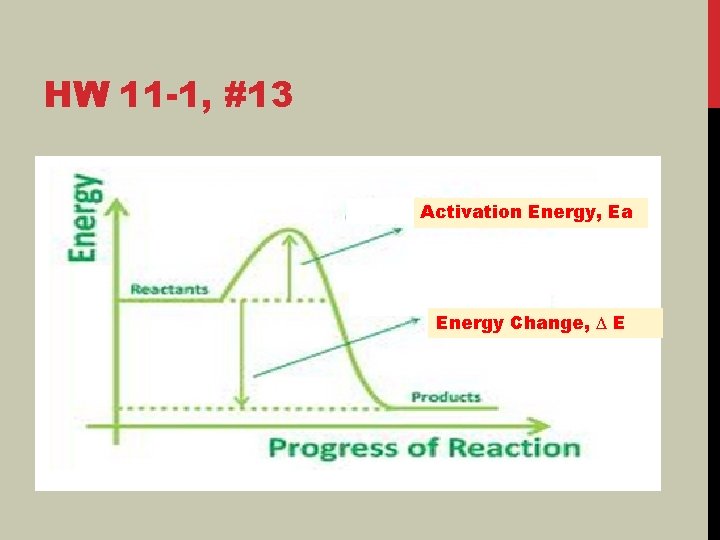
HW 11 -1, #13 Activation Energy, Ea Energy Change, ∆ E

HW 11 -1, #14 2 6

HEIGHT OF EA DETERMINES RXN RATE; THE LOWER THE EA, THE GREATER THE NUMBER OF MOLECULES THAT HAVE ENERGY (E) ≥ EA LOWER EA = FASTER RXN