Chemical Kinetics How fast chemical reactions proceed How

![A B time rate = - rate = D[A] Dt D[B] Dt A B time rate = - rate = D[A] Dt D[B] Dt](https://slidetodoc.com/presentation_image_h/2b9a8d4d46513fffcd88e6f858b99e66/image-5.jpg)
![a. A + b. B rate = - 1 D[A] a Dt = - a. A + b. B rate = - 1 D[A] a Dt = -](https://slidetodoc.com/presentation_image_h/2b9a8d4d46513fffcd88e6f858b99e66/image-8.jpg)
![Pseudo-first order reaction [Br. O 3 -]o [Br-]o [H+]o Initial conc M Complete reaction Pseudo-first order reaction [Br. O 3 -]o [Br-]o [H+]o Initial conc M Complete reaction](https://slidetodoc.com/presentation_image_h/2b9a8d4d46513fffcd88e6f858b99e66/image-26.jpg)
- Slides: 45

Chemical Kinetics • How fast chemical reactions proceed • How chemical reactions occur



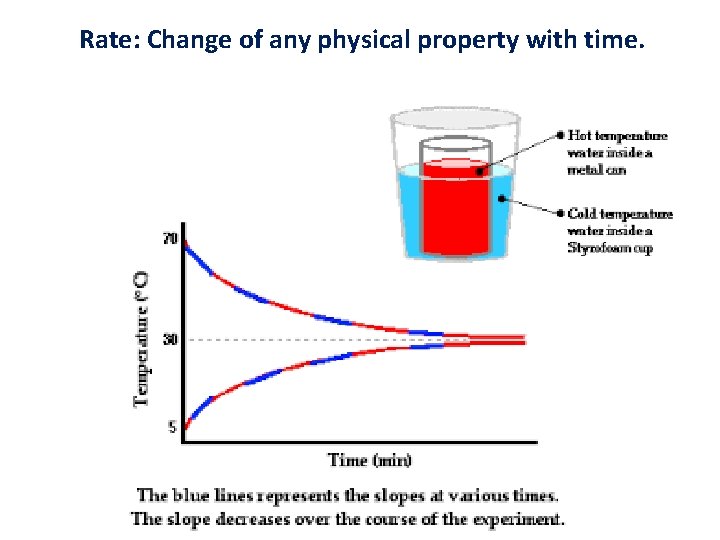
Rate: Change of any physical property with time.
![A B time rate rate DA Dt DB Dt A B time rate = - rate = D[A] Dt D[B] Dt](https://slidetodoc.com/presentation_image_h/2b9a8d4d46513fffcd88e6f858b99e66/image-5.jpg)
A B time rate = - rate = D[A] Dt D[B] Dt

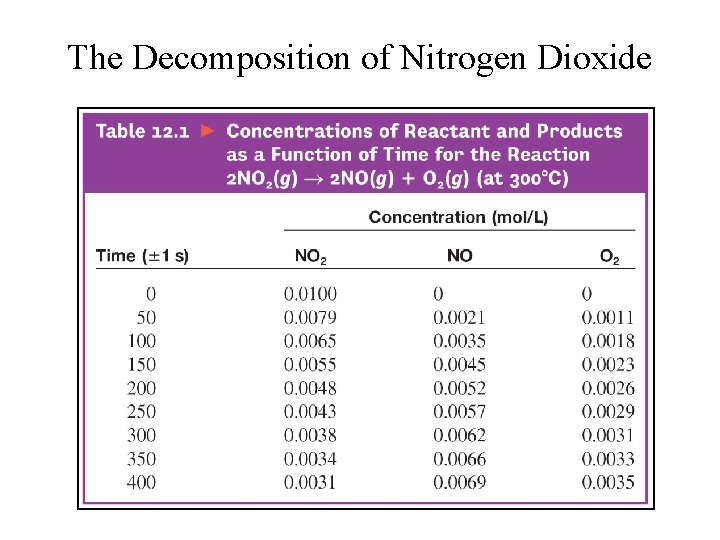
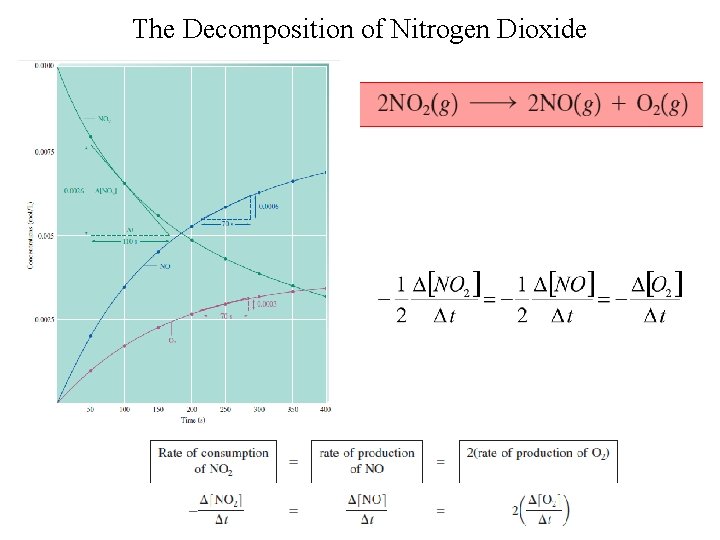
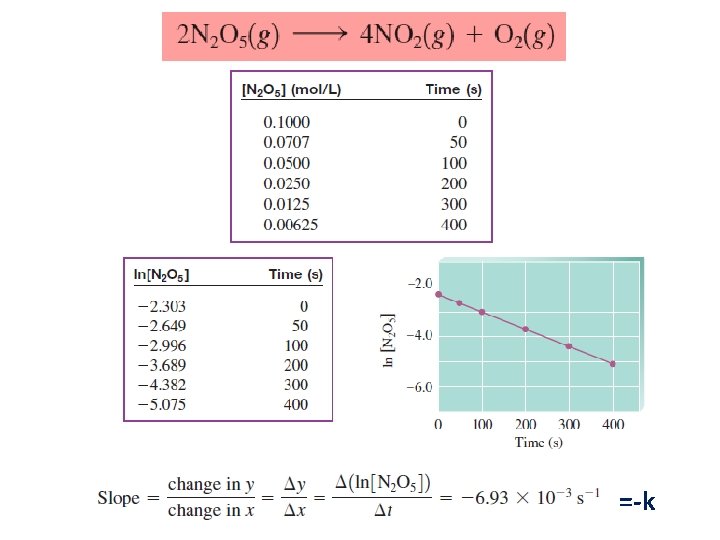
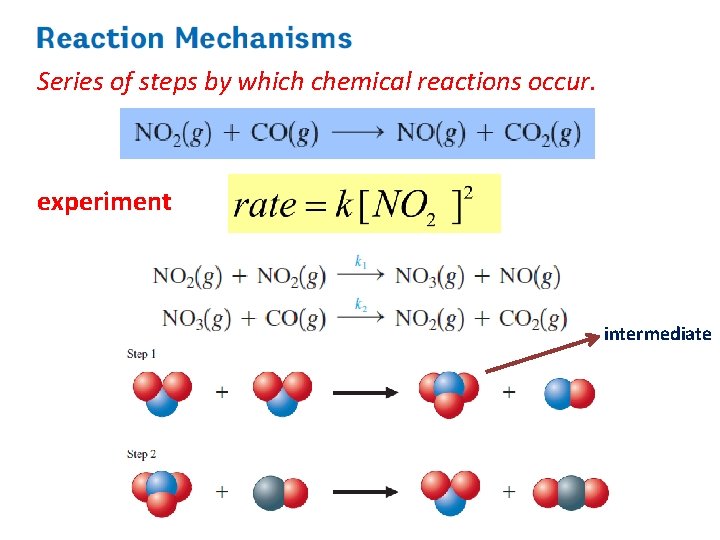
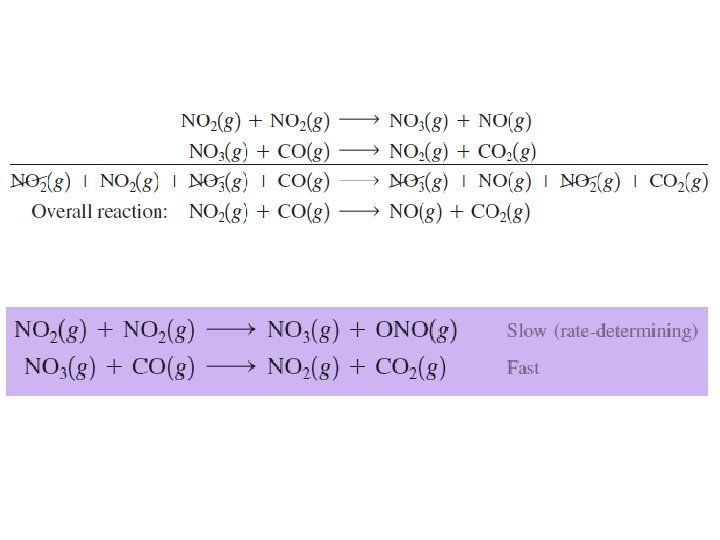
The Decomposition of Nitrogen Dioxide

The Decomposition of Nitrogen Dioxide
![a A b B rate 1 DA a Dt a. A + b. B rate = - 1 D[A] a Dt = -](https://slidetodoc.com/presentation_image_h/2b9a8d4d46513fffcd88e6f858b99e66/image-8.jpg)
a. A + b. B rate = - 1 D[A] a Dt = - c. C + d. D 1 D[B] b Dt = 1 D[C] c Dt = 1 D[D] d Dt

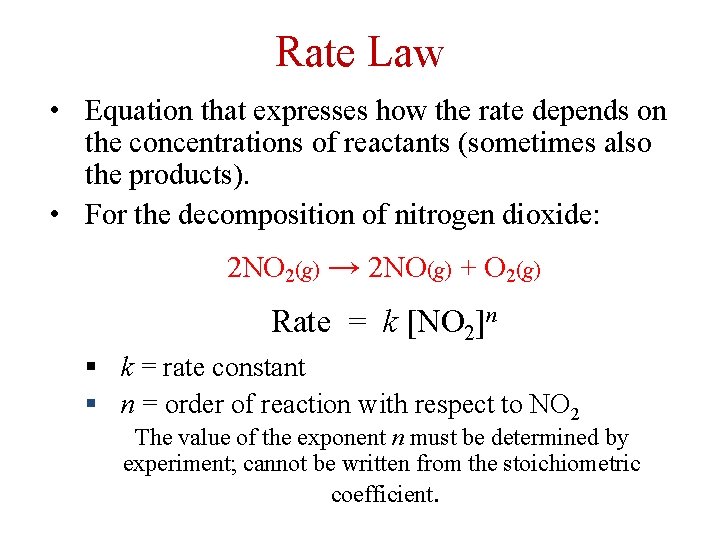
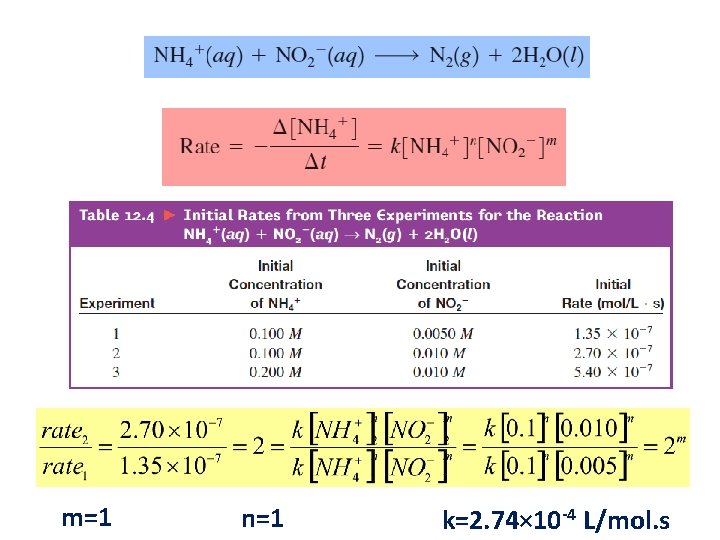
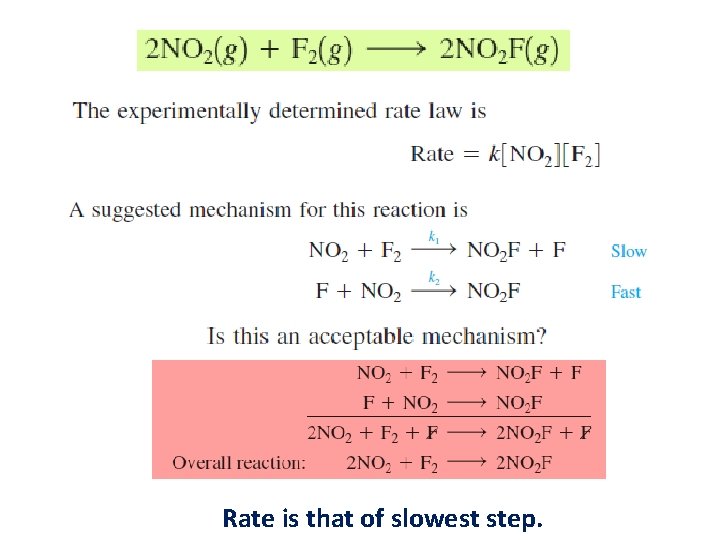
Rate Law • Equation that expresses how the rate depends on the concentrations of reactants (sometimes also the products). • For the decomposition of nitrogen dioxide: 2 NO 2(g) → 2 NO(g) + O 2(g) Rate = k [NO 2]n § k = rate constant § n = order of reaction with respect to NO 2 The value of the exponent n must be determined by experiment; cannot be written from the stoichiometric coefficient.

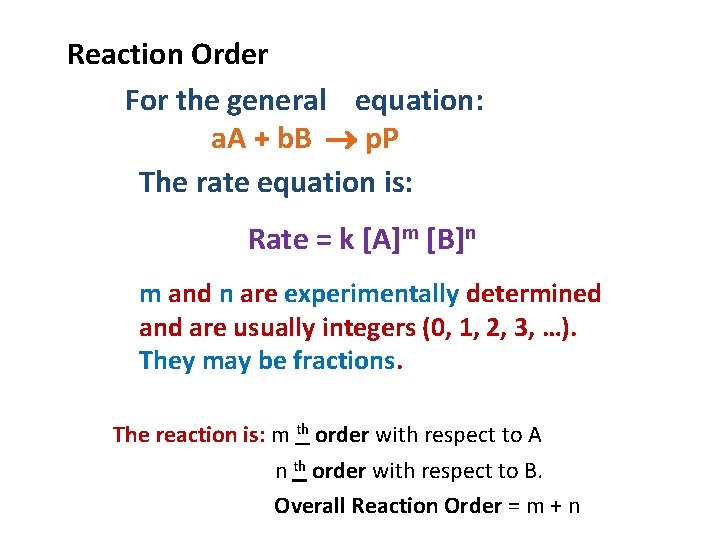
Reaction Order For the general equation: a. A + b. B p. P The rate equation is: Rate = k [A]m [B]n m and n are experimentally determined and are usually integers (0, 1, 2, 3, …). They may be fractions. The reaction is: m th order with respect to A n th order with respect to B. Overall Reaction Order = m + n

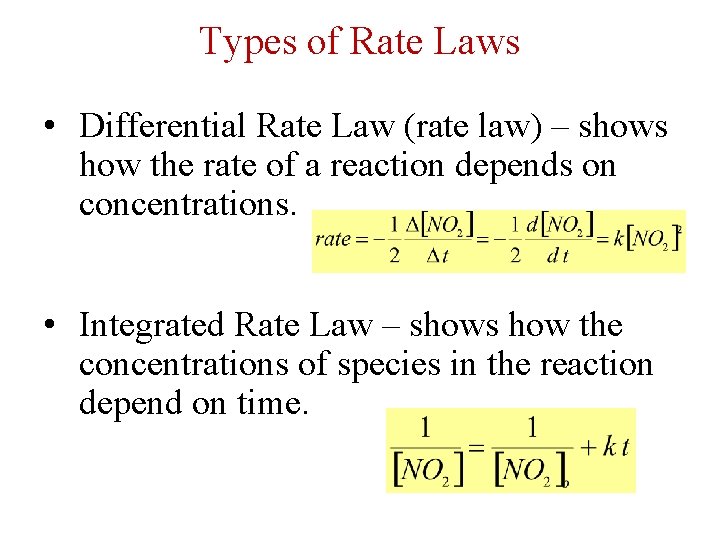
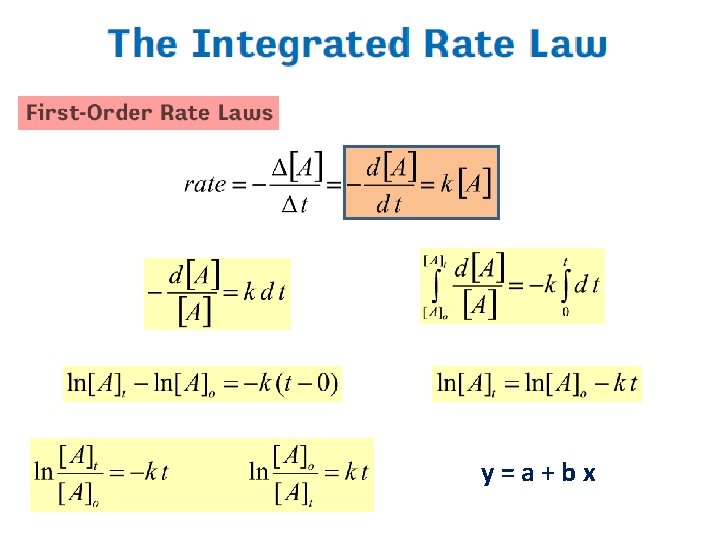
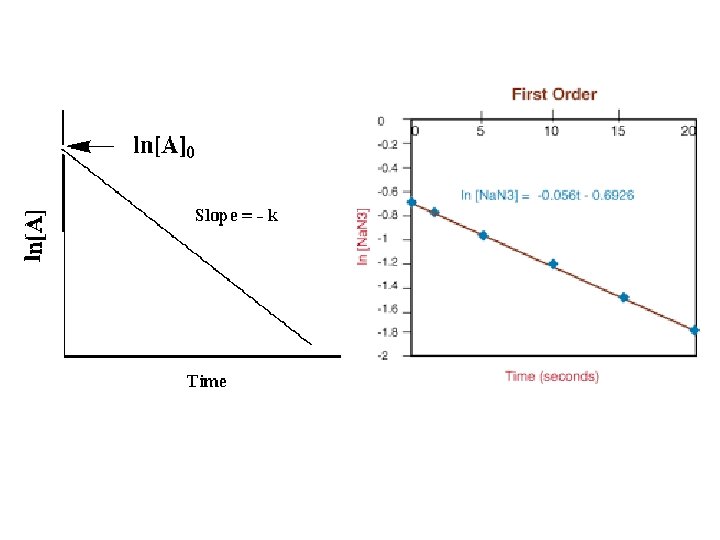
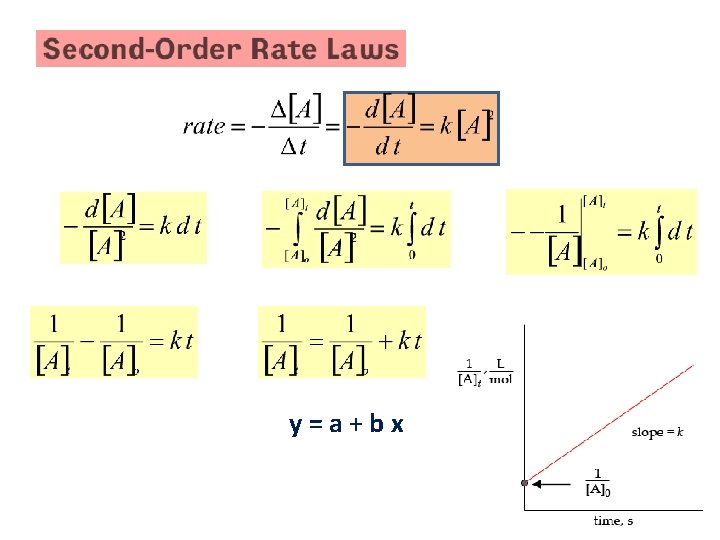
Types of Rate Laws • Differential Rate Law (rate law) – shows how the rate of a reaction depends on concentrations. • Integrated Rate Law – shows how the concentrations of species in the reaction depend on time.

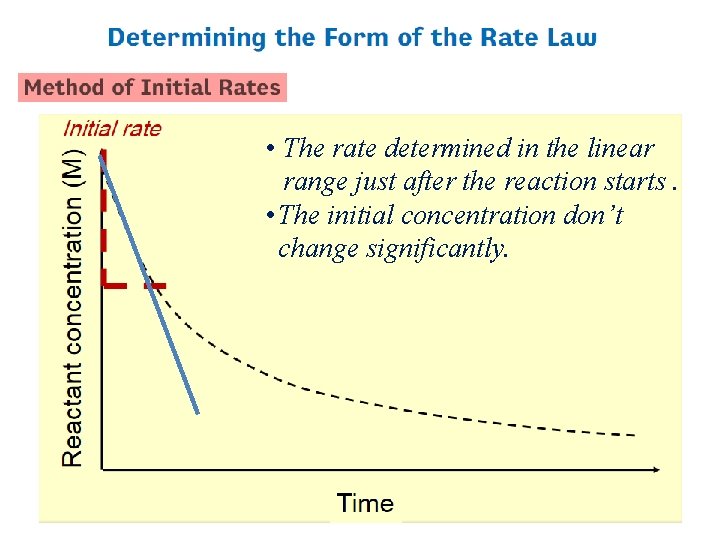
• The rate determined in the linear range just after the reaction starts. • The initial concentration don’t change significantly.

m=1 n=1 k=2. 74× 10 -4 L/mol. s

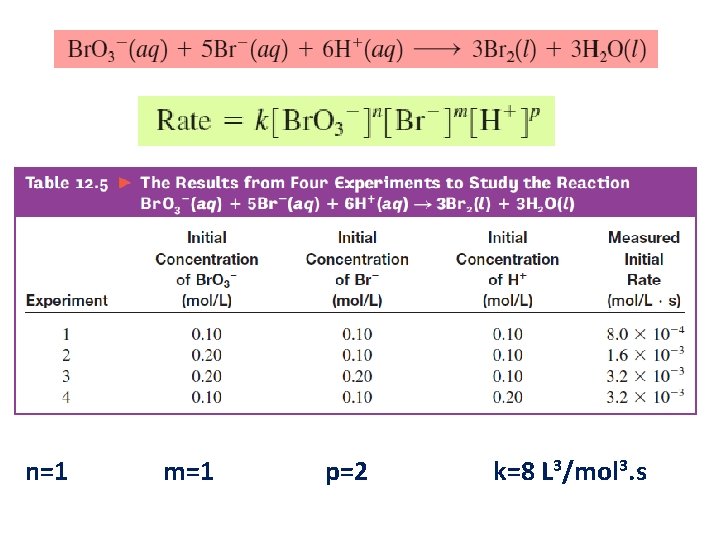
n=1 m=1 p=2 k=8 L 3/mol 3. s

y=a+bx



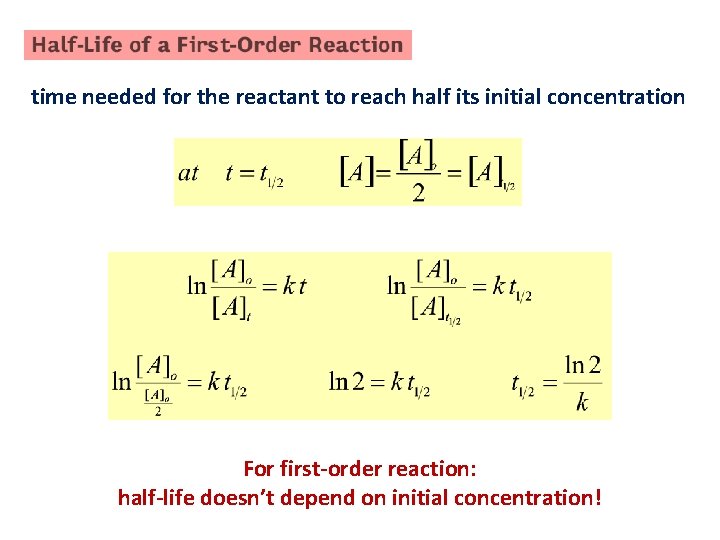
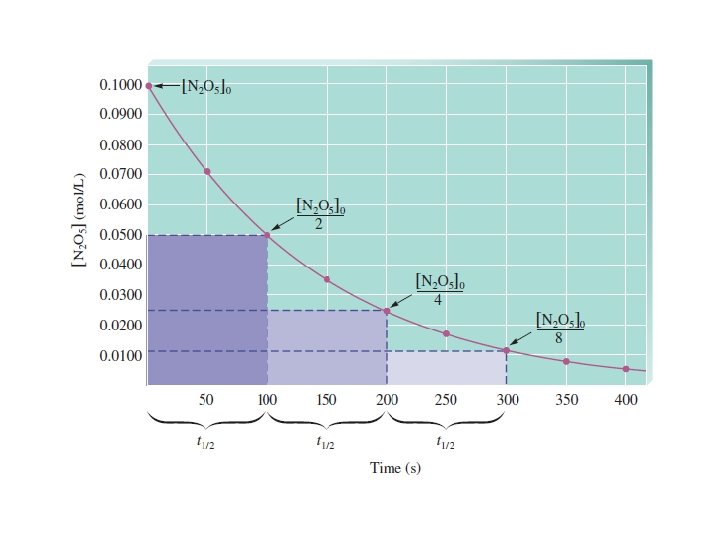
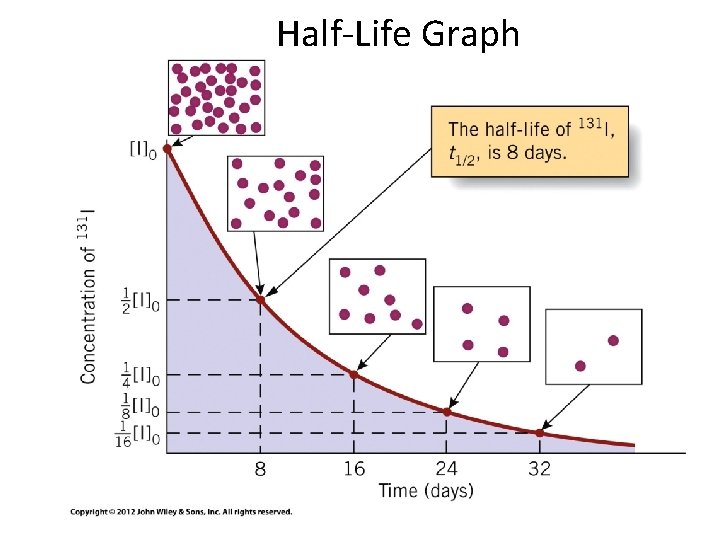
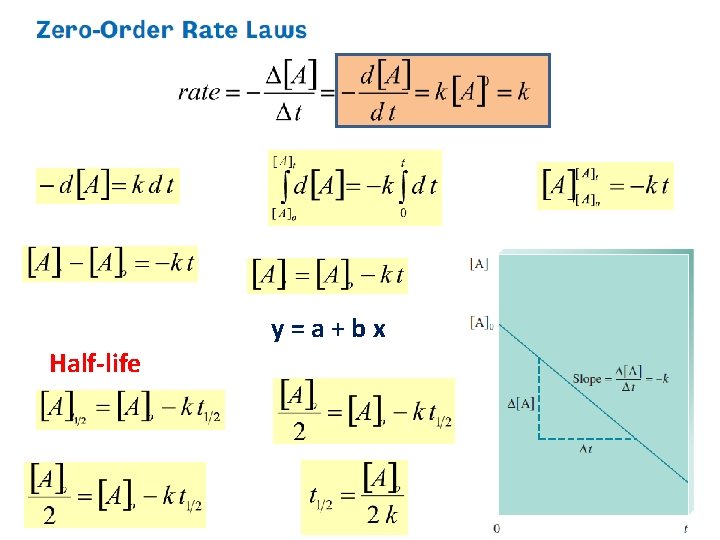
time needed for the reactant to reach half its initial concentration For first-order reaction: half-life doesn’t depend on initial concentration!


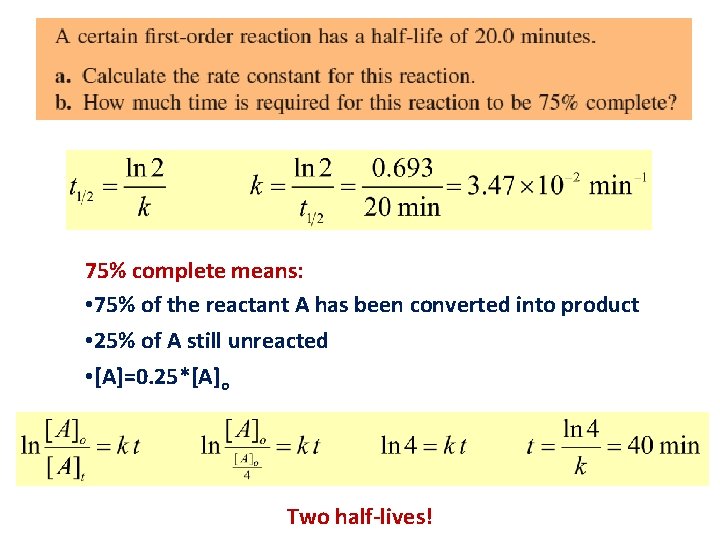
75% complete means: • 75% of the reactant A has been converted into product • 25% of A still unreacted • [A]=0. 25*[A]o Two half-lives!

Half-Life Graph 21

y=a+bx

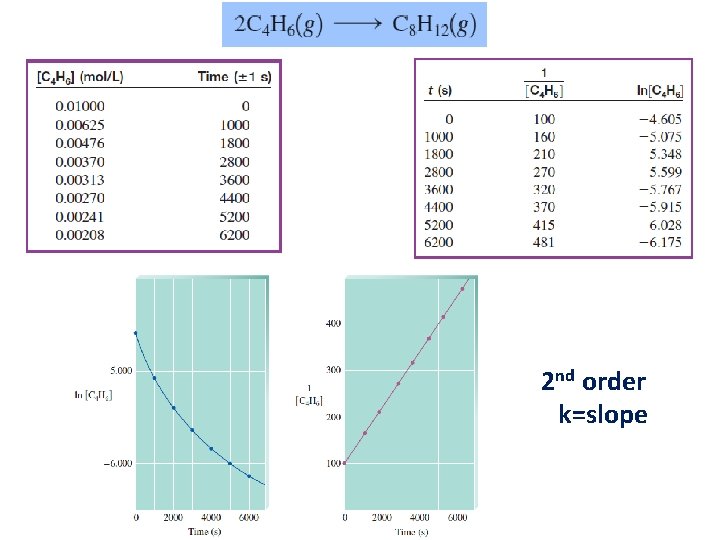
2 nd order k=slope

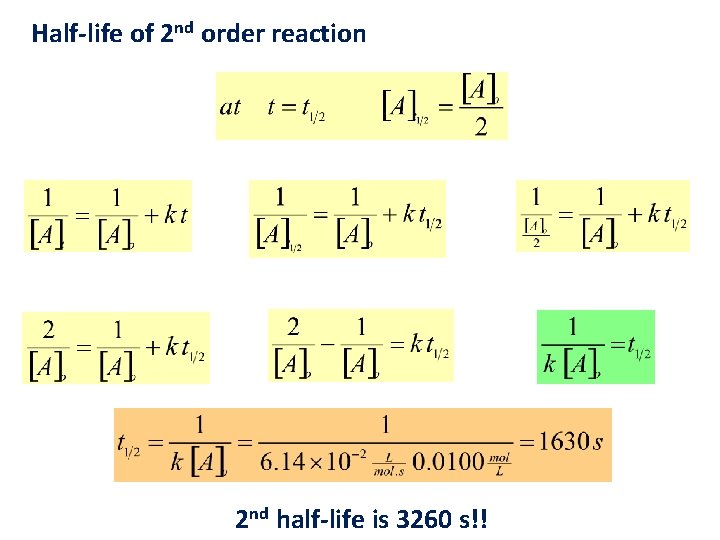
Half-life of 2 nd order reaction 2 nd half-life is 3260 s!!

y=a+bx Half-life
![Pseudofirst order reaction Br O 3 o Bro Ho Initial conc M Complete reaction Pseudo-first order reaction [Br. O 3 -]o [Br-]o [H+]o Initial conc M Complete reaction](https://slidetodoc.com/presentation_image_h/2b9a8d4d46513fffcd88e6f858b99e66/image-26.jpg)
Pseudo-first order reaction [Br. O 3 -]o [Br-]o [H+]o Initial conc M Complete reaction 1. 0× 10 -3 1. 0 0 0. 995 0. 994 k’ constant

Series of steps by which chemical reactions occur. experiment intermediate

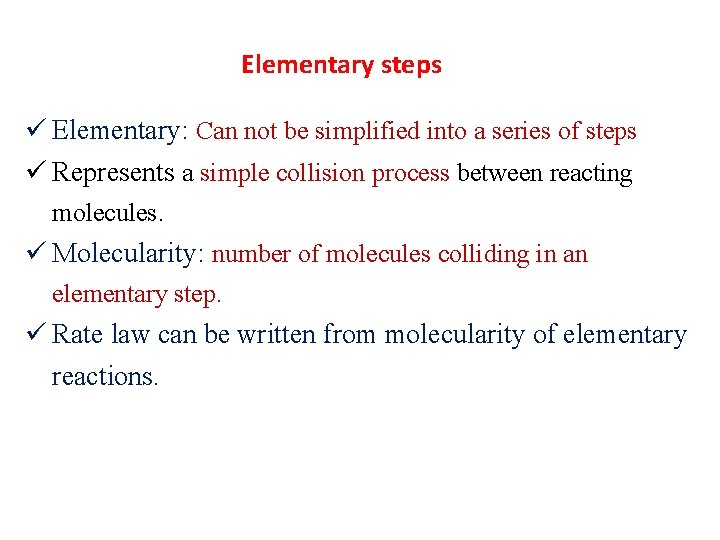
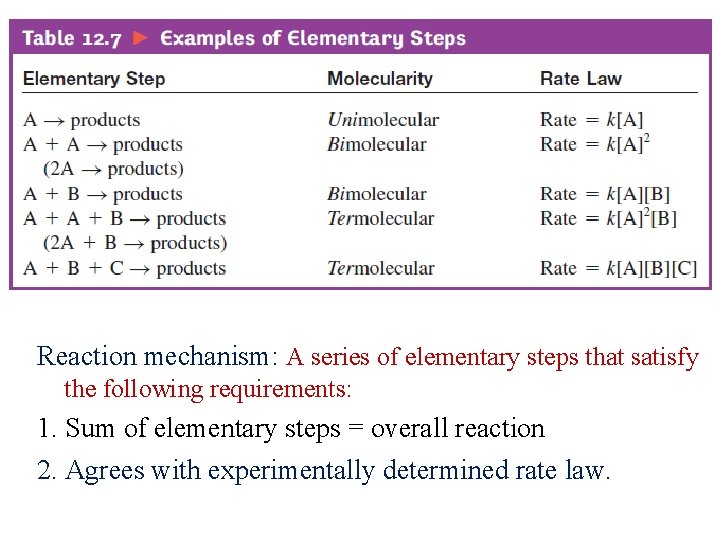
Elementary steps ü Elementary: Can not be simplified into a series of steps ü Represents a simple collision process between reacting molecules. ü Molecularity: number of molecules colliding in an elementary step. ü Rate law can be written from molecularity of elementary reactions.

Reaction mechanism: A series of elementary steps that satisfy the following requirements: 1. Sum of elementary steps = overall reaction 2. Agrees with experimentally determined rate law.


Rate is that of slowest step.

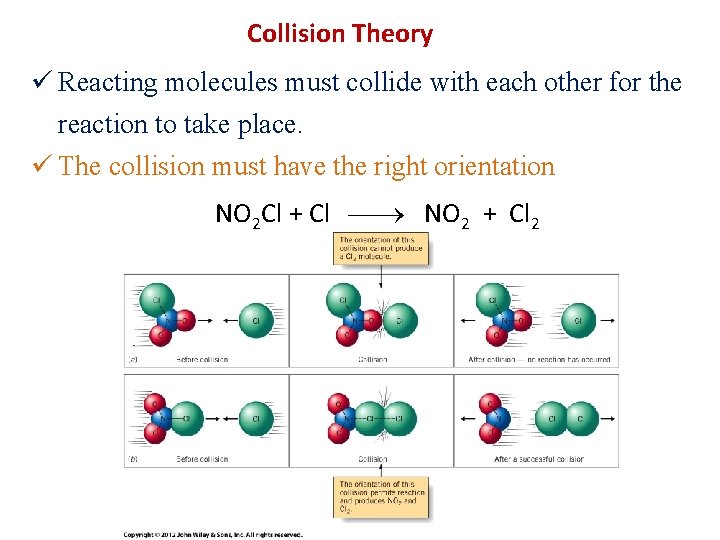
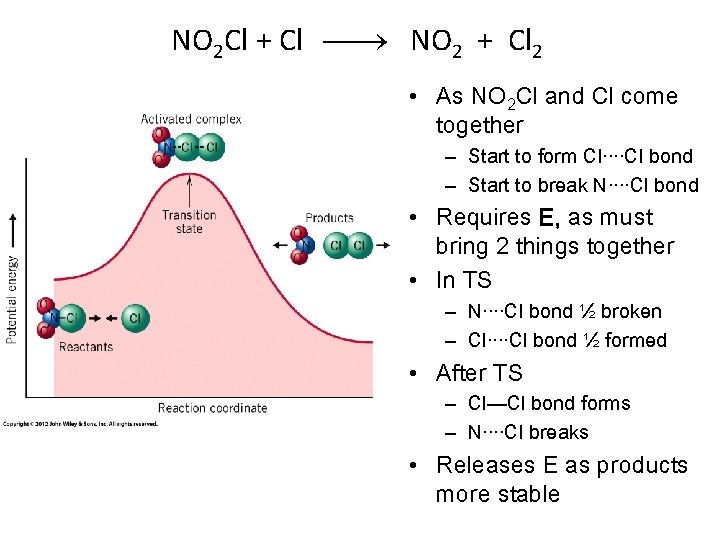
Collision Theory ü Reacting molecules must collide with each other for the reaction to take place. ü The collision must have the right orientation NO 2 Cl + Cl NO 2 + Cl 2

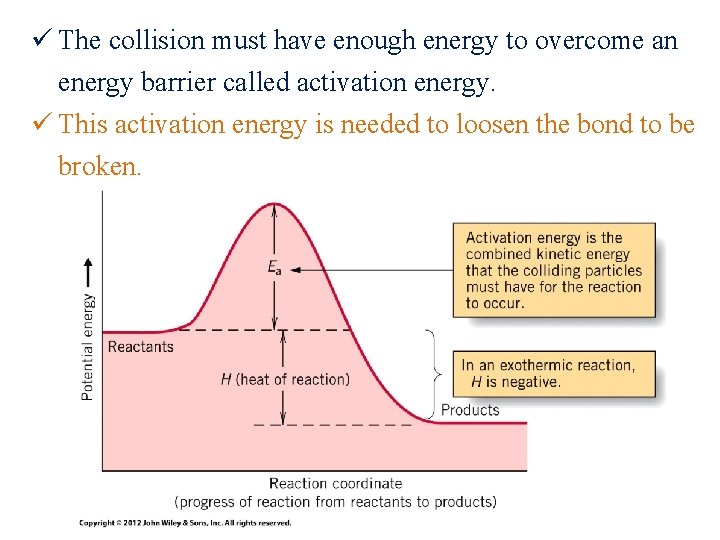
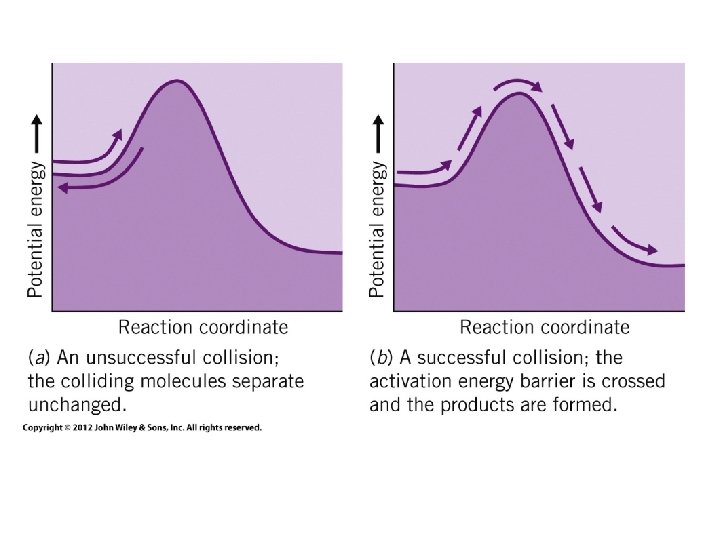
ü The collision must have enough energy to overcome an energy barrier called activation energy. ü This activation energy is needed to loosen the bond to be broken.

NO 2 Cl + Cl NO 2 + Cl 2 • As NO 2 Cl and Cl come together – Start to form Cl····Cl bond – Start to break N····Cl bond • Requires E, as must bring 2 things together • In TS – N····Cl bond ½ broken – Cl····Cl bond ½ formed • After TS – Cl—Cl bond forms – N····Cl breaks • Releases E as products more stable


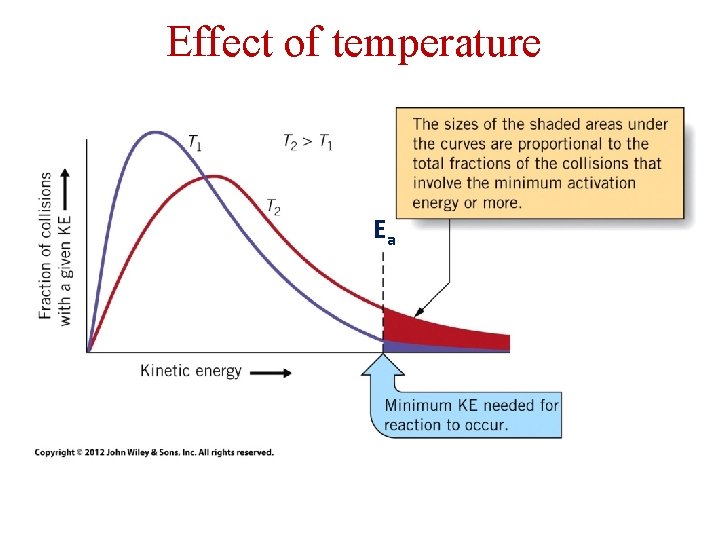
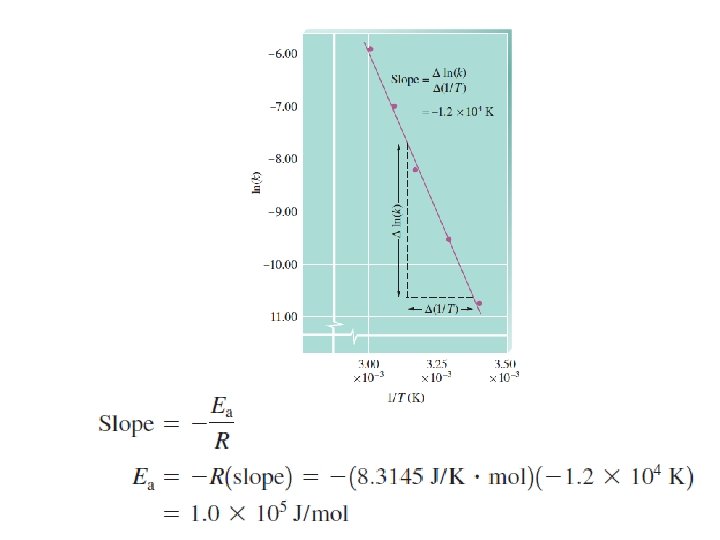
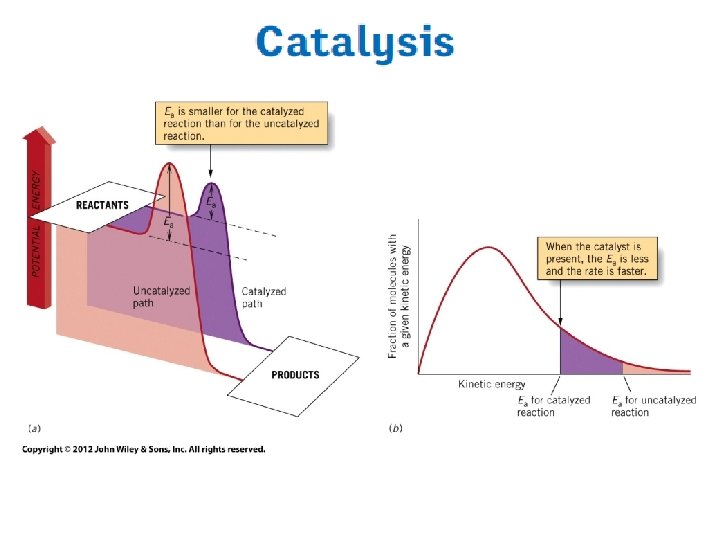
Effect of temperature Ea

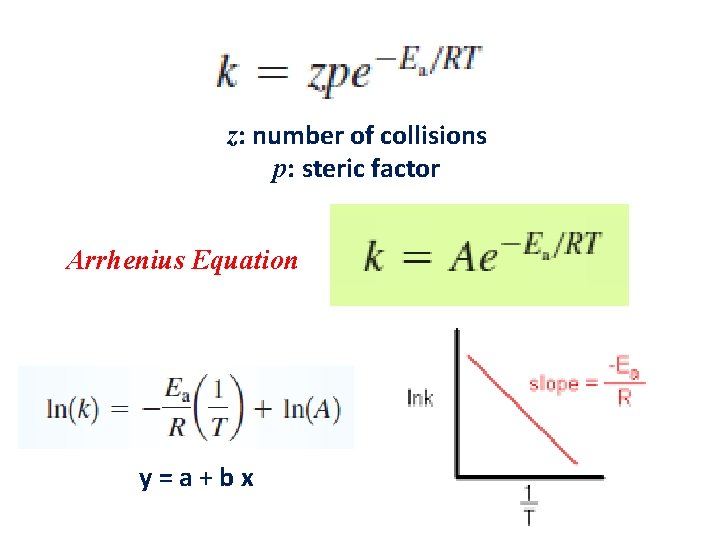
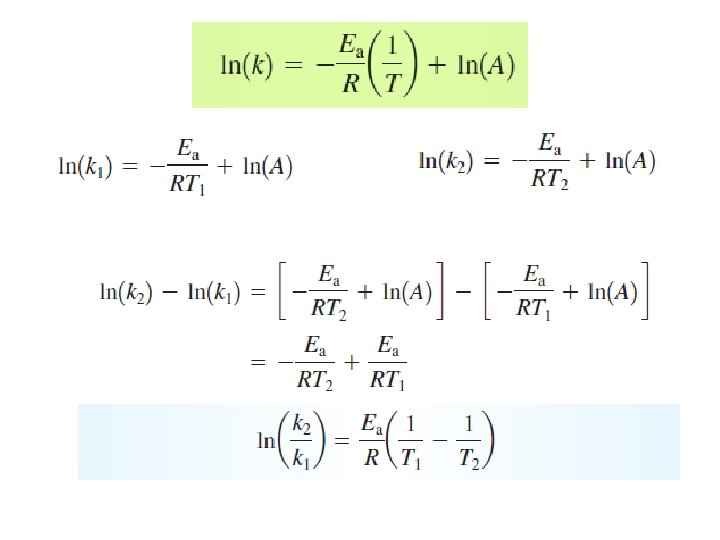
z: number of collisions p: steric factor Arrhenius Equation y=a+bx

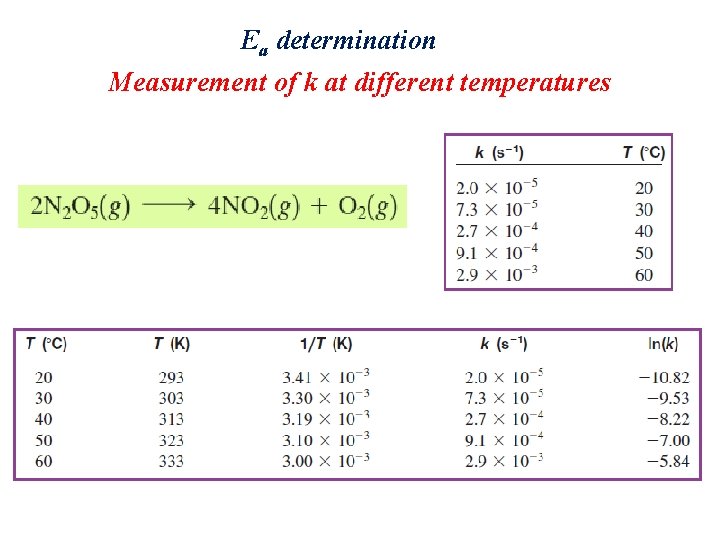
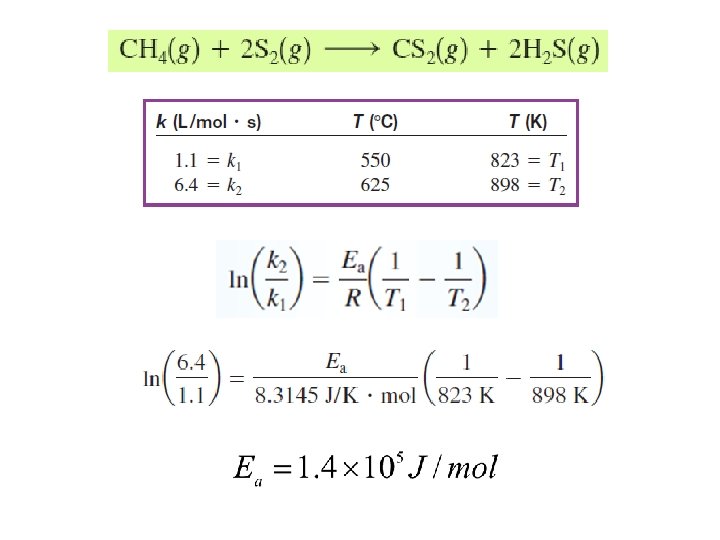
Ea determination Measurement of k at different temperatures




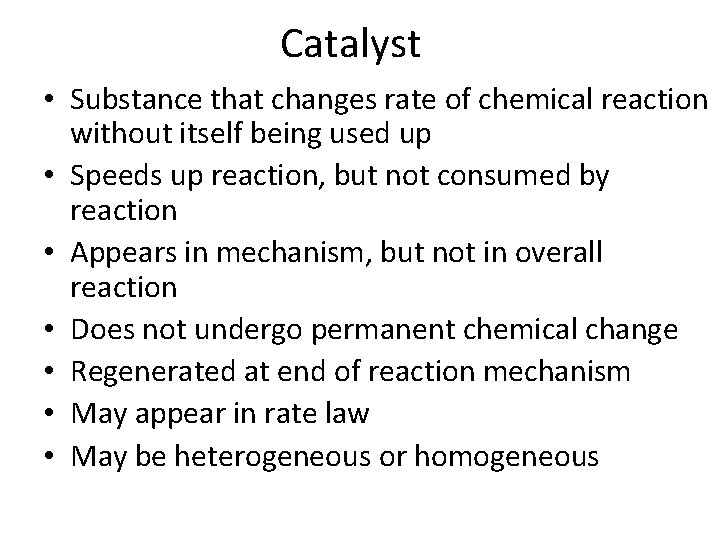
Catalyst • Substance that changes rate of chemical reaction without itself being used up • Speeds up reaction, but not consumed by reaction • Appears in mechanism, but not in overall reaction • Does not undergo permanent chemical change • Regenerated at end of reaction mechanism • May appear in rate law • May be heterogeneous or homogeneous


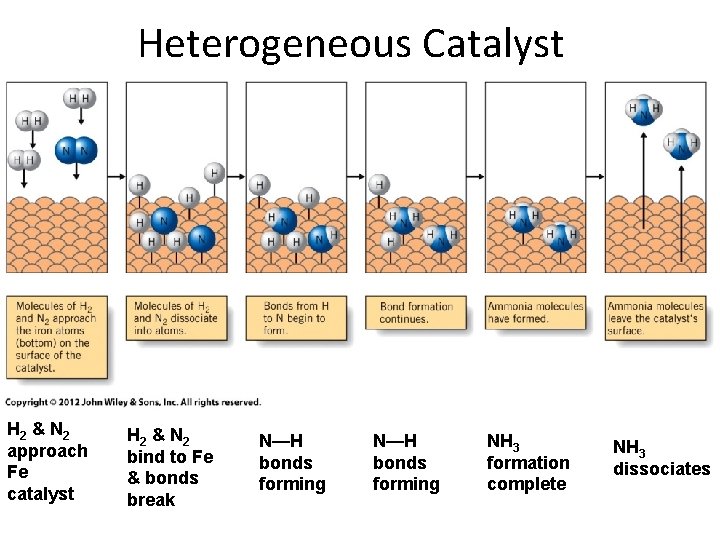
Heterogeneous Catalyst H 2 & N 2 approach Fe catalyst H 2 & N 2 bind to Fe & bonds break N—H bonds forming NH 3 formation complete NH 3 dissociates

Enzymes Acid rain