CHEMICAL KINETICS CHAPTER 17 Kinetics Fall 2009 CHEM


![mth order in [A] nth order in [B] 8 mth order in [A] nth order in [B] 8](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-8.jpg)
![[A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x [A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-9.jpg)
![[A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x [A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-10.jpg)

![INTEGRATED RATE LAWS Zero Order Reactions [A] - [Ao] = -kt Graph [A] vs INTEGRATED RATE LAWS Zero Order Reactions [A] - [Ao] = -kt Graph [A] vs](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-16.jpg)
![INTEGRATED RATE LAWS First Order Reactions ln[A]-ln[Ao] = -kt Graph ln[A] vs t Slope INTEGRATED RATE LAWS First Order Reactions ln[A]-ln[Ao] = -kt Graph ln[A] vs t Slope](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-17.jpg)
![Dimerization Data set provided [C 4 H 6] vs time 2 C 4 H Dimerization Data set provided [C 4 H 6] vs time 2 C 4 H](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-19.jpg)


![Need to express [intermediates] in terms of other reactants 29 Need to express [intermediates] in terms of other reactants 29](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-29.jpg)
![Substituting for [N 2 O 2] in the rate expression above 30 Substituting for [N 2 O 2] in the rate expression above 30](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-30.jpg)



- Slides: 60

CHEMICAL KINETICS CHAPTER 17, Kinetics Fall 2009, CHEM 1310 1

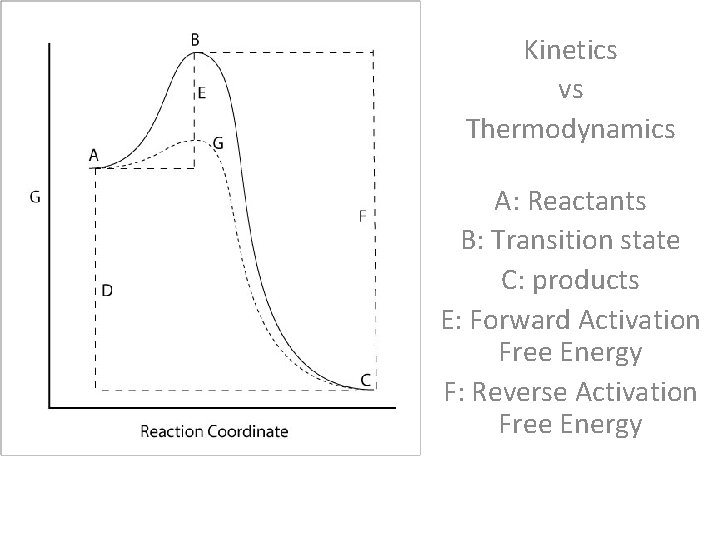
Kinetics vs Thermodynamics Ke. Kin A: Reactants B: Transition state C: products E: Forward Activation Free Energy F: Reverse Activation Free Energy

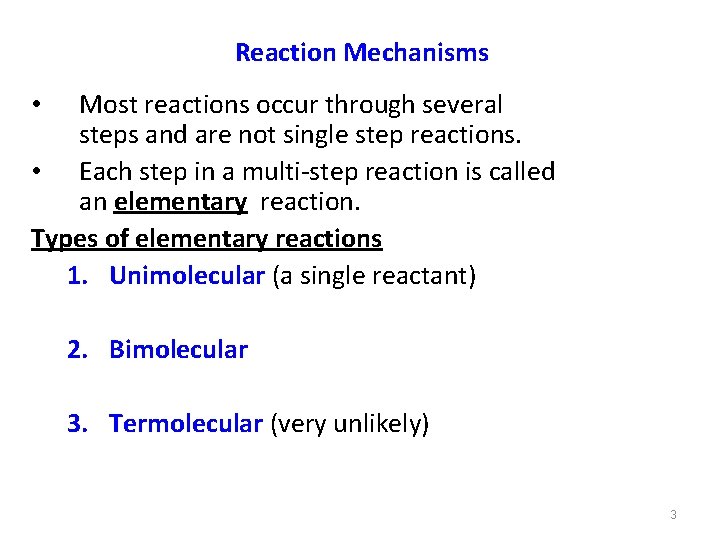
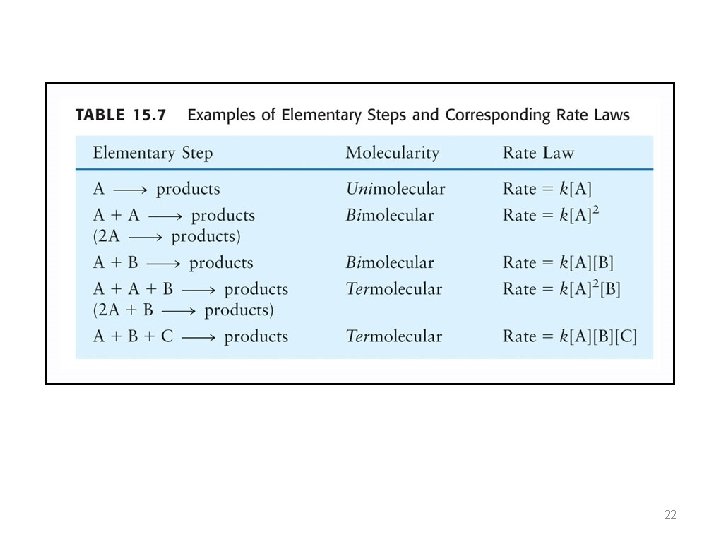
Reaction Mechanisms Most reactions occur through several steps and are not single step reactions. • Each step in a multi-step reaction is called an elementary reaction. Types of elementary reactions 1. Unimolecular (a single reactant) • 2. Bimolecular 3. Termolecular (very unlikely) 3

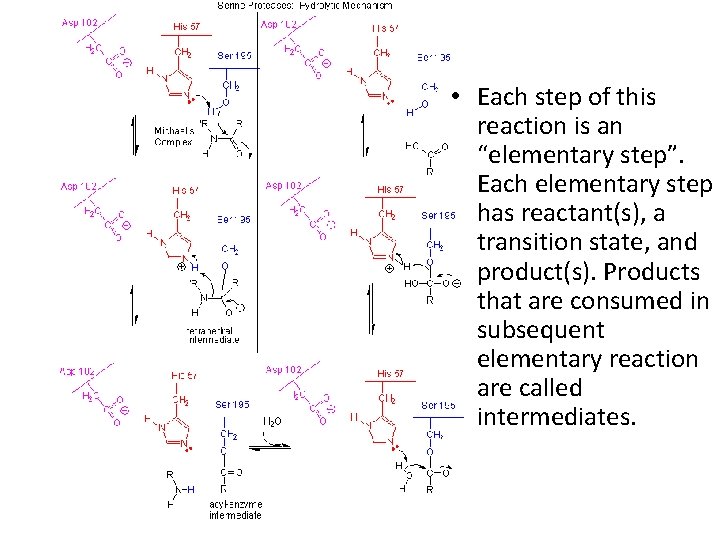
• Each step of this reaction is an “elementary step”. Each elementary step has reactant(s), a transition state, and product(s). Products that are consumed in subsequent elementary reaction are called intermediates.

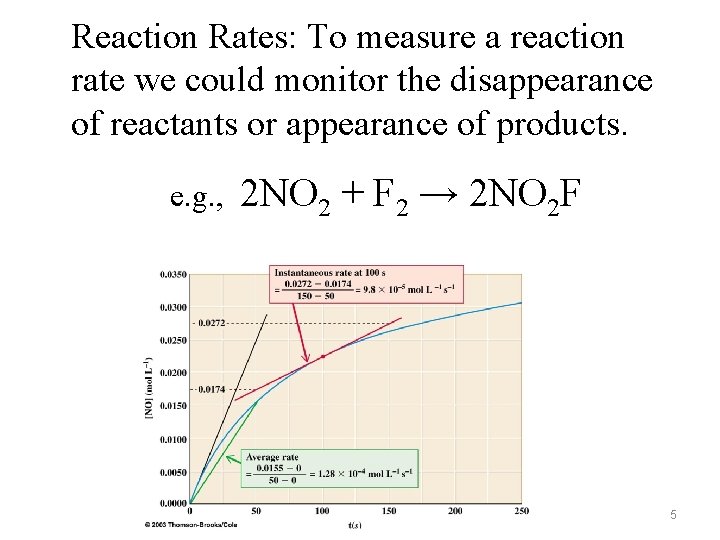
Reaction Rates: To measure a reaction rate we could monitor the disappearance of reactants or appearance of products. e. g. , 2 NO 2 + F 2 → 2 NO 2 F 5

Gen. Rxn: a. A + b. B → c. C + d. D NO 2 + CO → NO + CO 2 6

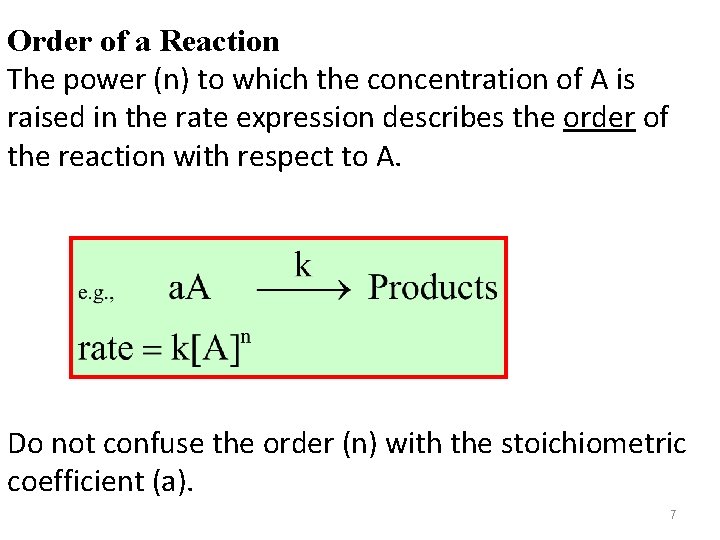
Order of a Reaction The power (n) to which the concentration of A is raised in the rate expression describes the order of the reaction with respect to A. Do not confuse the order (n) with the stoichiometric coefficient (a). 7
![mth order in A nth order in B 8 mth order in [A] nth order in [B] 8](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-8.jpg)
mth order in [A] nth order in [B] 8
![A mol L1 B mol L1 Rate mol L1 s1 1 1 0 x [A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-9.jpg)
[A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x 10 -4 1. 0 x 10 -4 2. 8 x 10 -6 2 1. 0 x 10 -4 3. 0 x 10 -4 8. 4 x 10 -6 3 2. 0 x 10 -4 3. 0 x 10 -4 3. 4 x 10 -5 9
![A mol L1 B mol L1 Rate mol L1 s1 1 1 0 x [A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-10.jpg)
[A] (mol L-1) [B] (mol L-1) Rate (mol L-1 s-1) 1 1. 0 x 10 -4 1. 0 x 10 -4 2. 8 x 10 -6 2 1. 0 x 10 -4 3. 0 x 10 -4 8. 4 x 10 -6 3 2. 0 x 10 -4 3. 0 x 10 -4 3. 4 x 10 -5 10

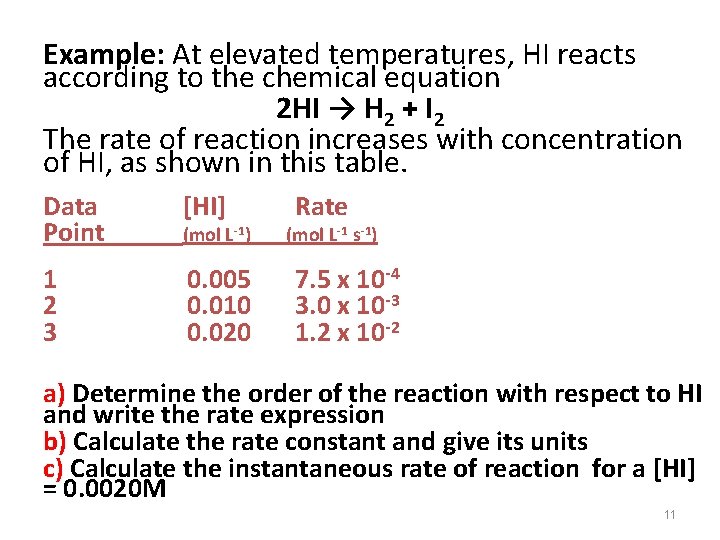
Example: At elevated temperatures, HI reacts according to the chemical equation 2 HI → H 2 + I 2 The rate of reaction increases with concentration of HI, as shown in this table. Data Point [HI] 1 2 3 0. 005 0. 010 0. 020 (mol L-1) Rate (mol L-1 s-1) 7. 5 x 10 -4 3. 0 x 10 -3 1. 2 x 10 -2 a) Determine the order of the reaction with respect to HI and write the rate expression b) Calculate the rate constant and give its units c) Calculate the instantaneous rate of reaction for a [HI] = 0. 0020 M 11

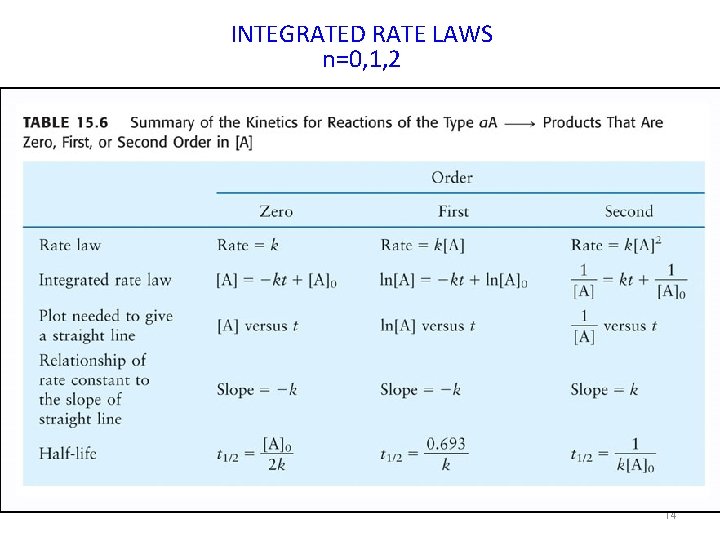
INTEGRATED RATE LAWS • Single Reactant (three cases) – Zero-Order Rate Law (n = 0) – First-Order Rate Law (n = 1) – Second-Order Rate Law (n = 2) • More than one Reactant – Must state the order of the reaction with respect to each reactant (rate = k[A]n[B]m[C]p) 12

13

INTEGRATED RATE LAWS n=0, 1, 2 14

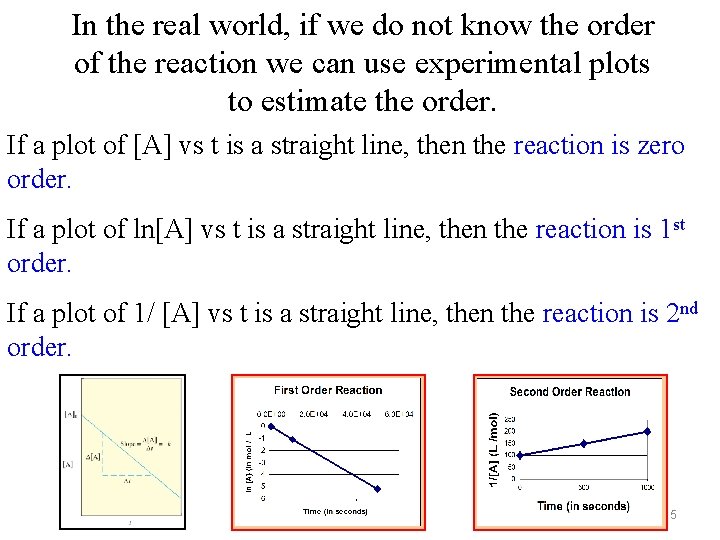
In the real world, if we do not know the order of the reaction we can use experimental plots to estimate the order. If a plot of [A] vs t is a straight line, then the reaction is zero order. If a plot of ln[A] vs t is a straight line, then the reaction is 1 st order. If a plot of 1/ [A] vs t is a straight line, then the reaction is 2 nd order. 15
![INTEGRATED RATE LAWS Zero Order Reactions A Ao kt Graph A vs INTEGRATED RATE LAWS Zero Order Reactions [A] - [Ao] = -kt Graph [A] vs](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-16.jpg)
INTEGRATED RATE LAWS Zero Order Reactions [A] - [Ao] = -kt Graph [A] vs t Slope = -k, intercept = [Ao] 16
![INTEGRATED RATE LAWS First Order Reactions lnAlnAo kt Graph lnA vs t Slope INTEGRATED RATE LAWS First Order Reactions ln[A]-ln[Ao] = -kt Graph ln[A] vs t Slope](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-17.jpg)
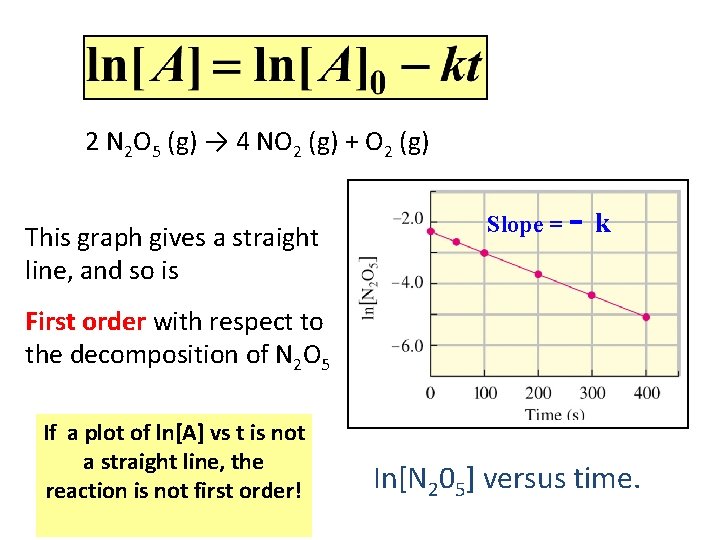
INTEGRATED RATE LAWS First Order Reactions ln[A]-ln[Ao] = -kt Graph ln[A] vs t Slope = -k, intercept = [Ao] 17

2 N 2 O 5 (g) → 4 NO 2 (g) + O 2 (g) This graph gives a straight line, and so is Slope = -k First order with respect to the decomposition of N 2 O 5 If a plot of ln[A] vs t is not a straight line, the reaction is not first order! In[N 205] versus time. 18
![Dimerization Data set provided C 4 H 6 vs time 2 C 4 H Dimerization Data set provided [C 4 H 6] vs time 2 C 4 H](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-19.jpg)
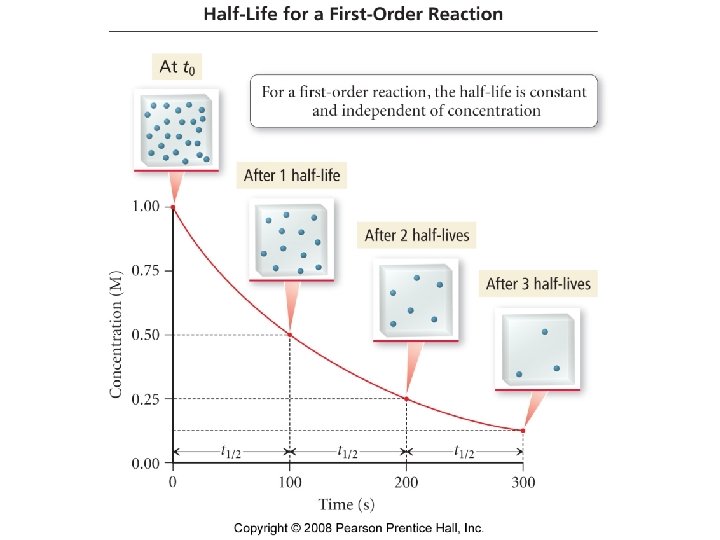
Dimerization Data set provided [C 4 H 6] vs time 2 C 4 H 6 (g) → C 8 H 12 (g) [C 4 H 6] = 0. 01 M ˚ 19


Reaction Mechanisms • • Most reactions proceed not through a single step but through a series of steps Each Step is called an elementary reaction Types of elementary reactions 1. Unimolecular (a single reactant) E. g. , A → B + C (a decomposition) 2. Bimolecular (most common type) E. g. , A + B → products 3. Termolecular (less likely event) E. g. , A + B + C → products 21

22

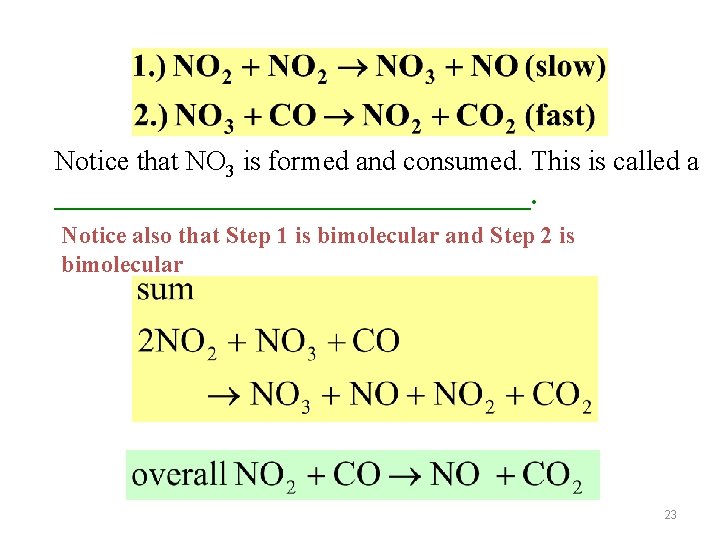
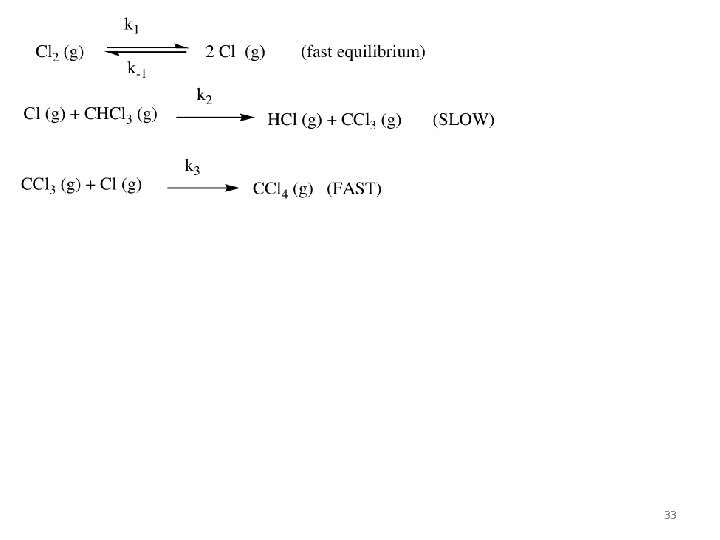
Notice that NO 3 is formed and consumed. This is called a _________________. Notice also that Step 1 is bimolecular and Step 2 is bimolecular 23

CHEMICAL EQUILIBRIUM A direct connection exists between the equilibrium constant of a reaction and the rate constants. a) at equilibrium: forward reaction rate = reverse reaction rate. b) Keq = kf / kr (same as K = k 1/k-1) kf A ⇌ kr B 24

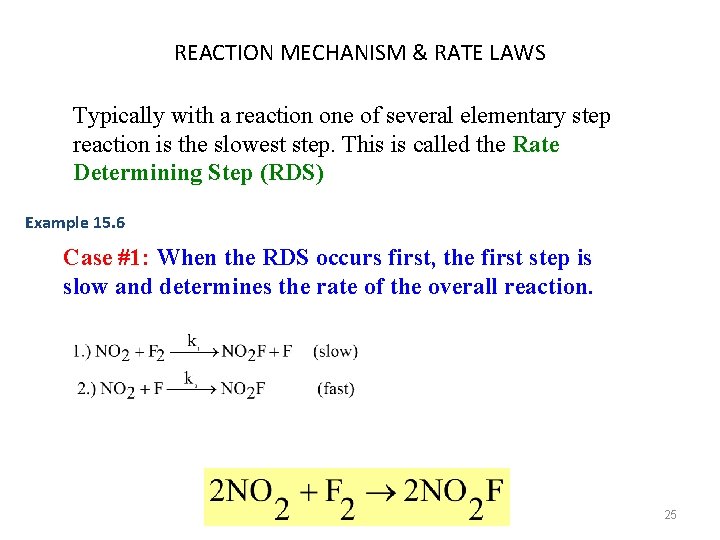
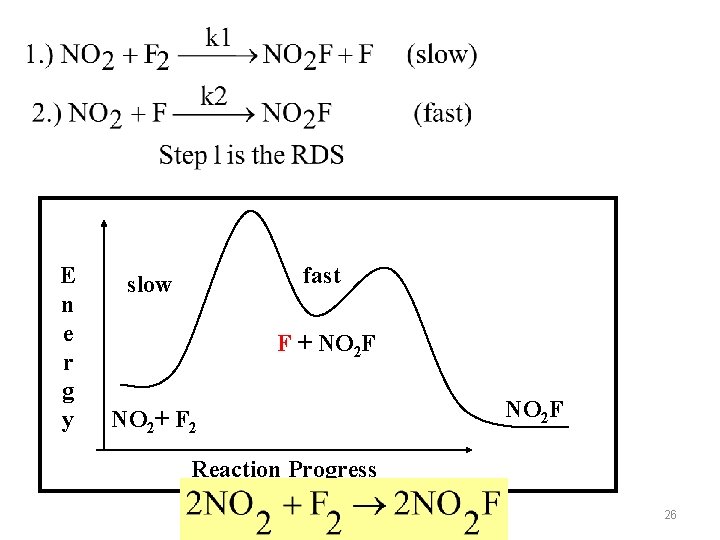
REACTION MECHANISM & RATE LAWS Typically with a reaction one of several elementary step reaction is the slowest step. This is called the Rate Determining Step (RDS) Example 15. 6 Case #1: When the RDS occurs first, the first step is slow and determines the rate of the overall reaction. 25

E n e r g y fast slow F + NO 2 F NO 2+ F 2 NO 2 F Reaction Progress 26

Chem 1310 Spring 2009 stop here

E n e r g y Reaction Progress 28
![Need to express intermediates in terms of other reactants 29 Need to express [intermediates] in terms of other reactants 29](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-29.jpg)
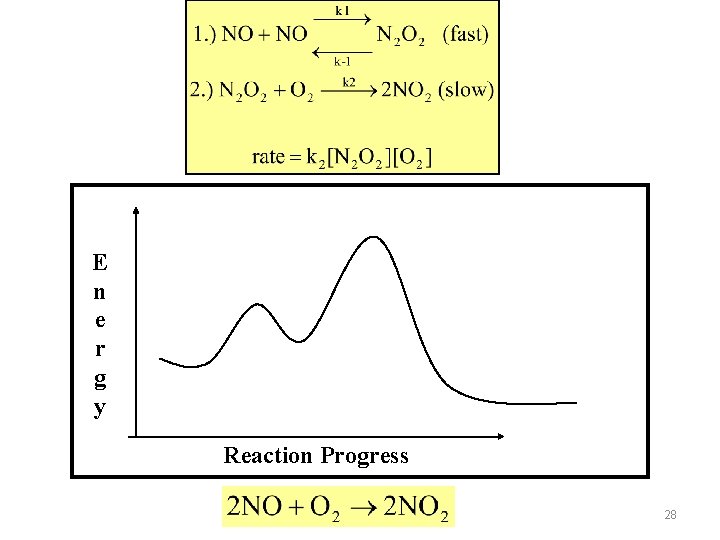
Need to express [intermediates] in terms of other reactants 29
![Substituting for N 2 O 2 in the rate expression above 30 Substituting for [N 2 O 2] in the rate expression above 30](https://slidetodoc.com/presentation_image_h/1c82dd84130295c42679f3defbe873a9/image-30.jpg)
Substituting for [N 2 O 2] in the rate expression above 30

E n e r g y slow fast N 2 O 2 + O 2 2 NO 2 Reaction Progress 31

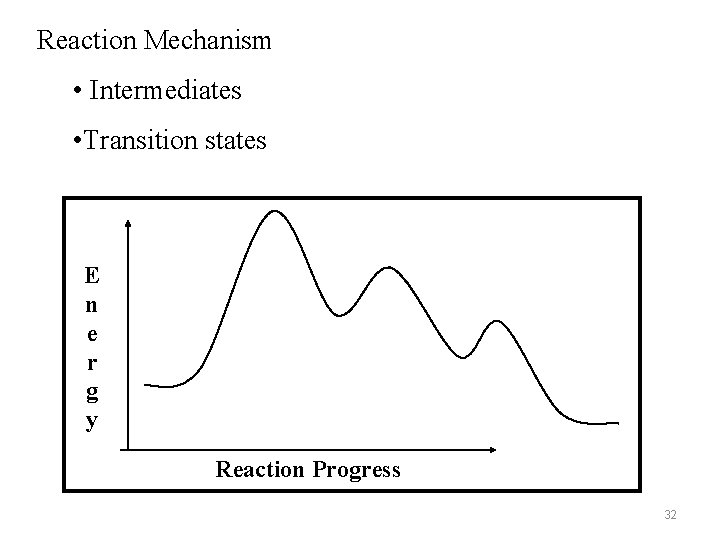
Reaction Mechanism • Intermediates • Transition states E n e r g y Reaction Progress 32

33

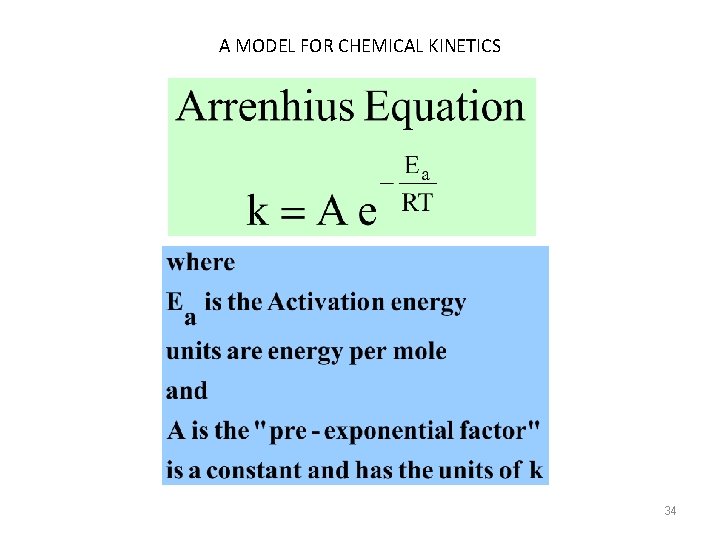
A MODEL FOR CHEMICAL KINETICS 34

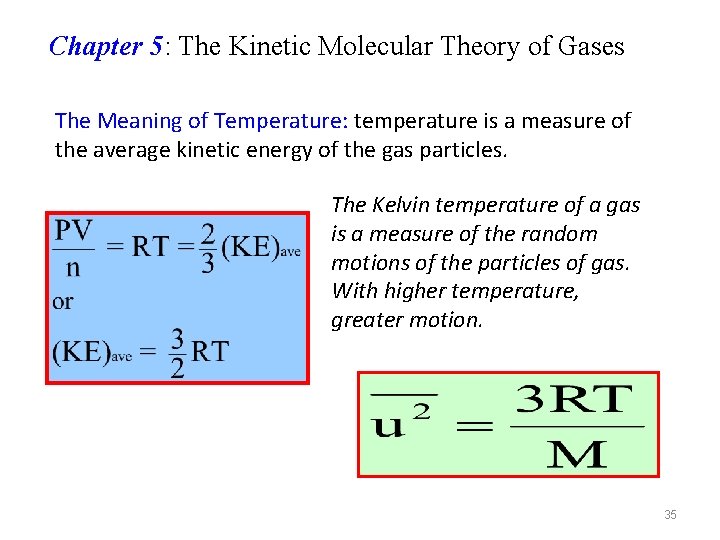
Chapter 5: The Kinetic Molecular Theory of Gases The Meaning of Temperature: temperature is a measure of the average kinetic energy of the gas particles. The Kelvin temperature of a gas is a measure of the random motions of the particles of gas. With higher temperature, greater motion. 35

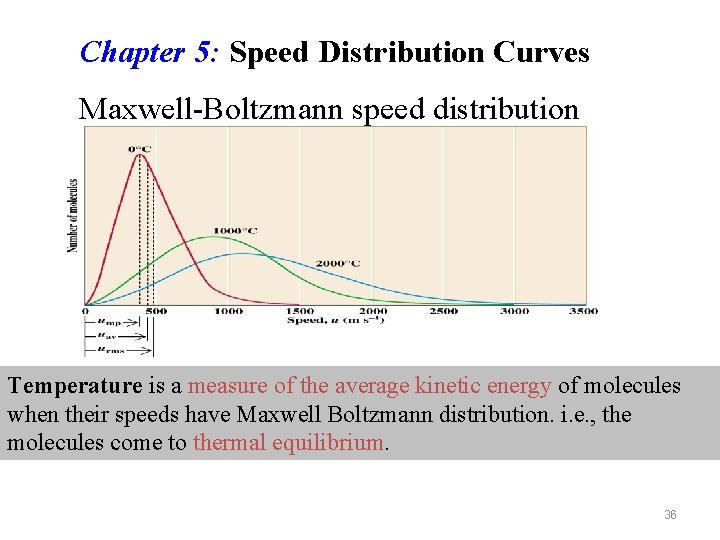
Chapter 5: Speed Distribution Curves Maxwell-Boltzmann speed distribution Temperature is a measure of the average kinetic energy of molecules when their speeds have Maxwell Boltzmann distribution. i. e. , the molecules come to thermal equilibrium. 36

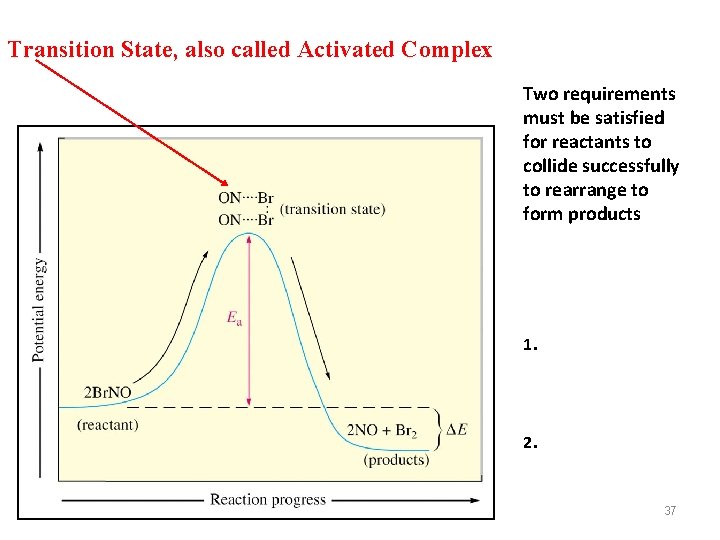
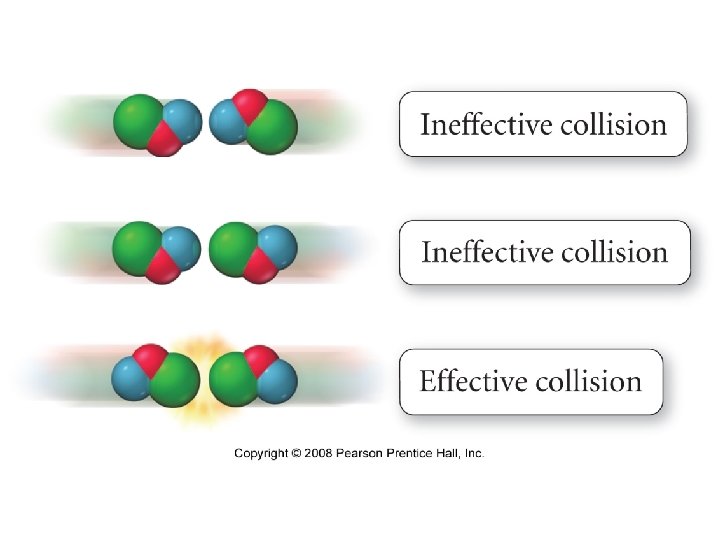
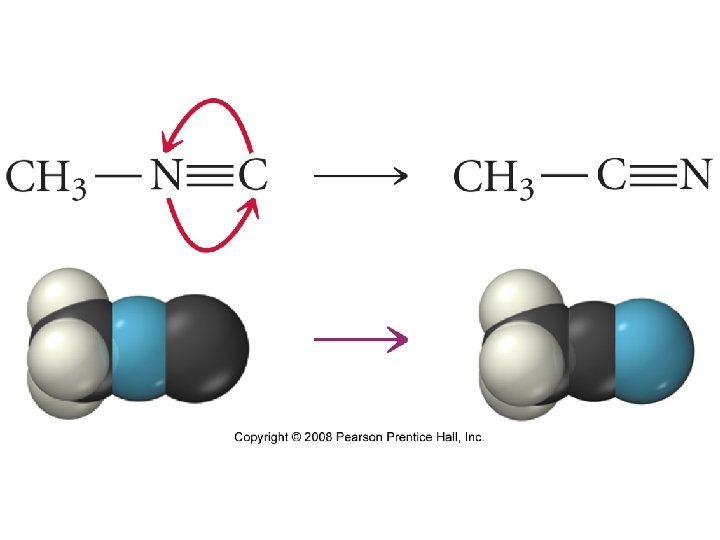
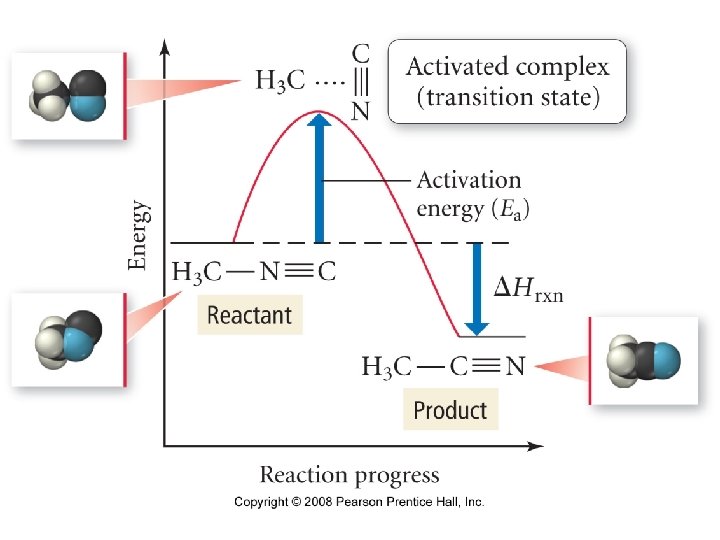
Transition State, also called Activated Complex Two requirements must be satisfied for reactants to collide successfully to rearrange to form products 1. 2. 37

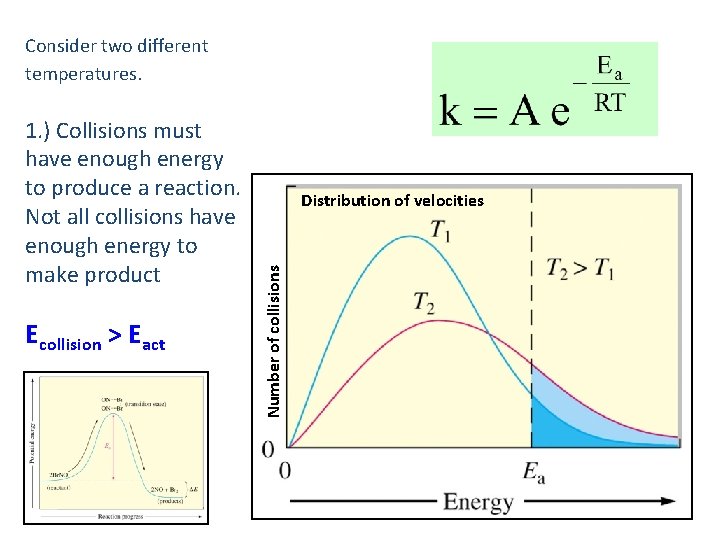
Consider two different temperatures. Ecollision > Eact Distribution of velocities Number of collisions 1. ) Collisions must have enough energy to produce a reaction. Not all collisions have enough energy to make product 38

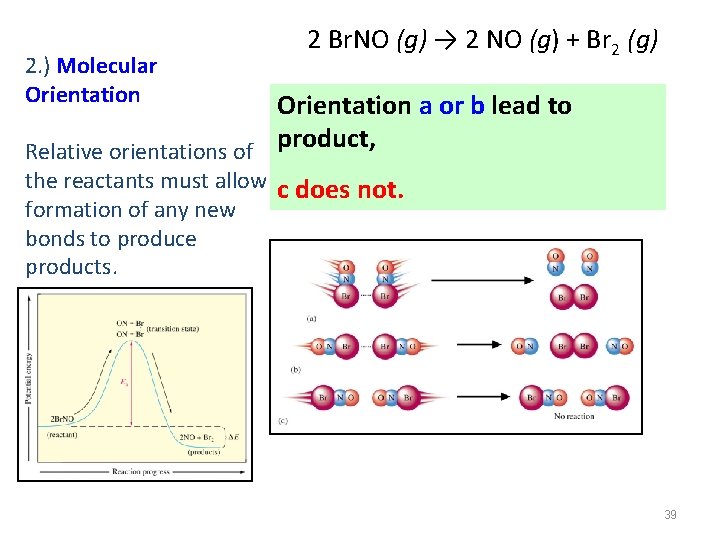
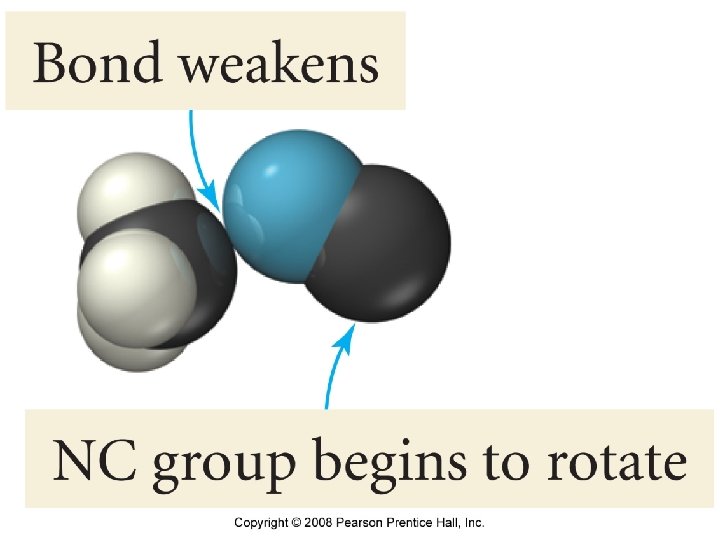
2. ) Molecular Orientation 2 Br. NO (g) → 2 NO (g) + Br 2 (g) Orientation a or b lead to Relative orientations of product, the reactants must allow c does not. formation of any new bonds to produce products. 39



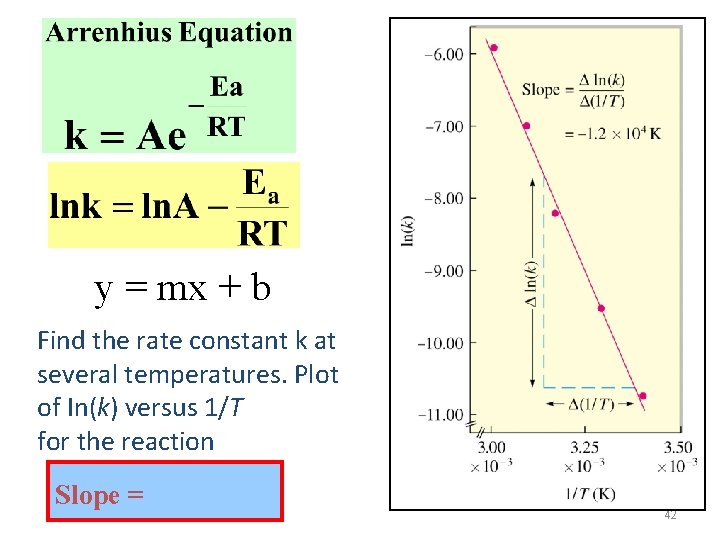
y = mx + b Find the rate constant k at several temperatures. Plot of In(k) versus 1/T for the reaction Slope = 42

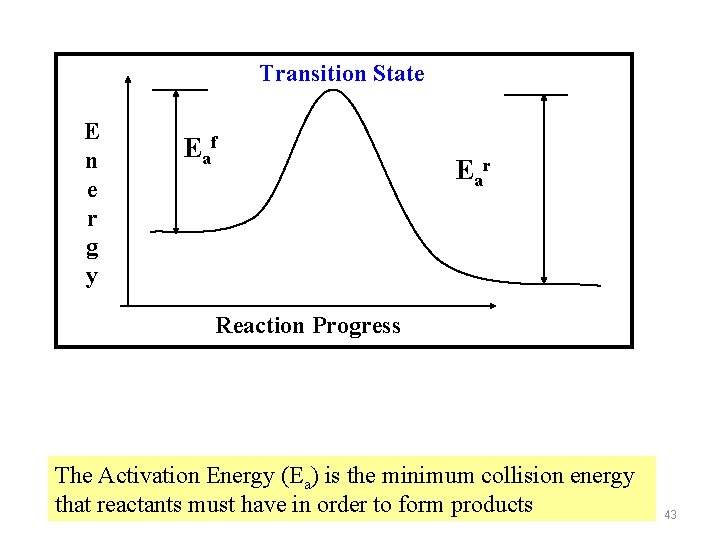
Transition State E n e r g y Eaf Ear Reaction Progress The Activation Energy (Ea) is the minimum collision energy that reactants must have in order to form products 43

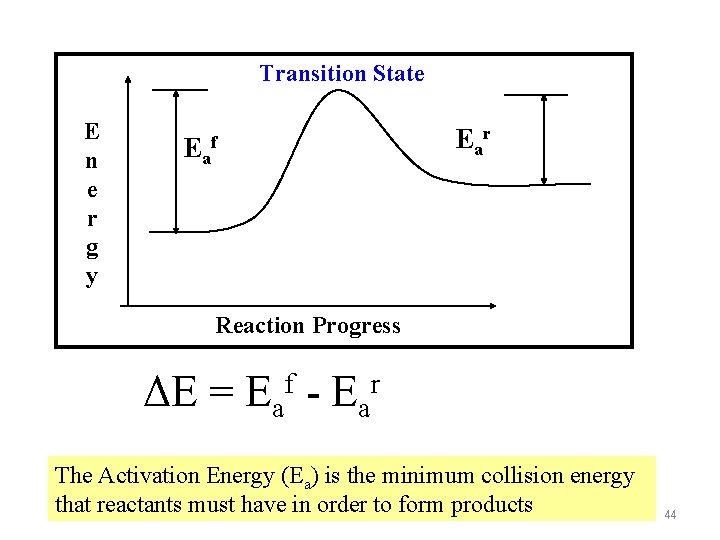
Transition State E n e r g y Eaf Ear Reaction Progress ΔE = Eaf - Ear The Activation Energy (Ea) is the minimum collision energy that reactants must have in order to form products 44




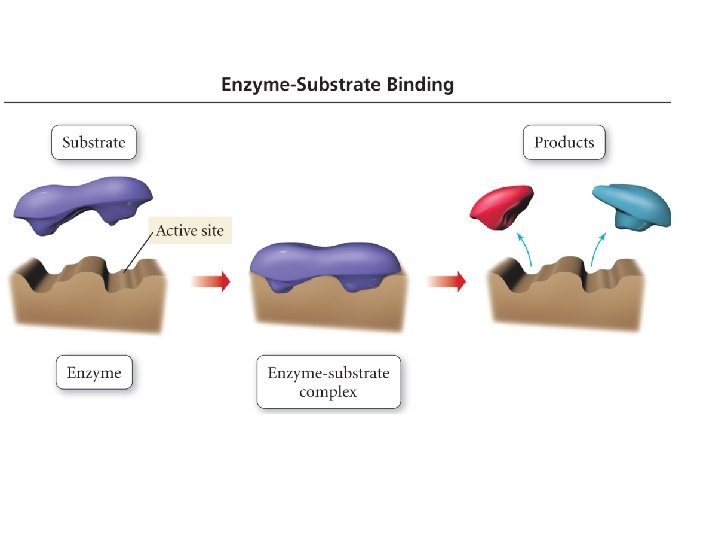
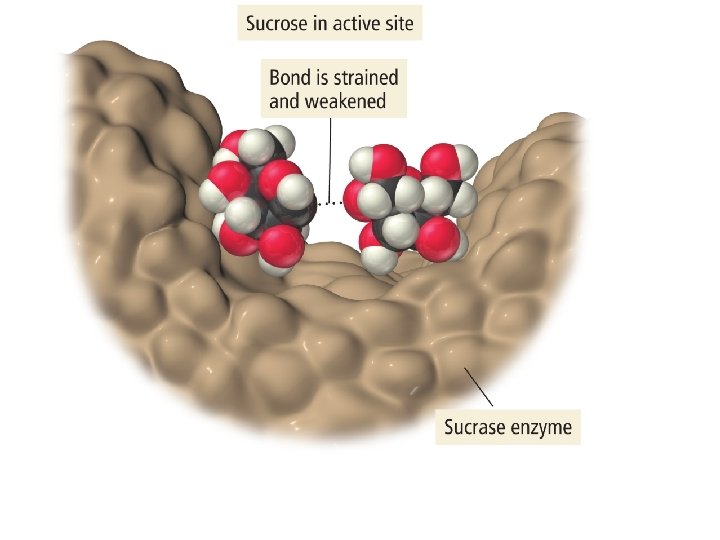
CHEMICAL KINETICS • Catalyst • Inhibitor 48

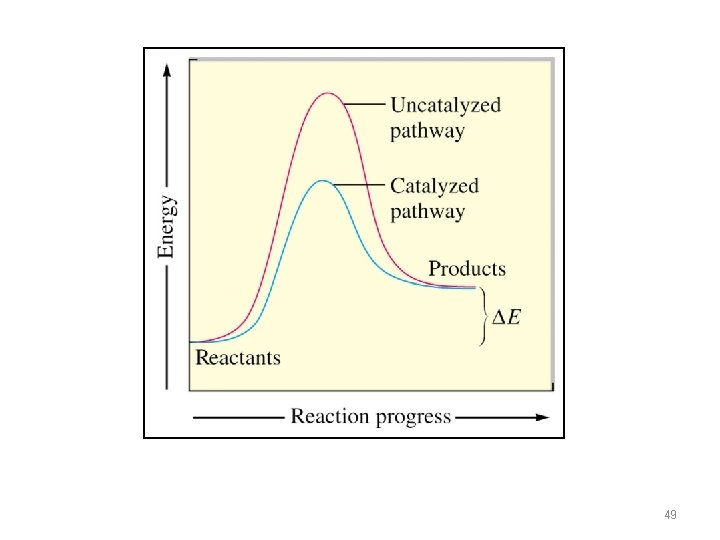
49


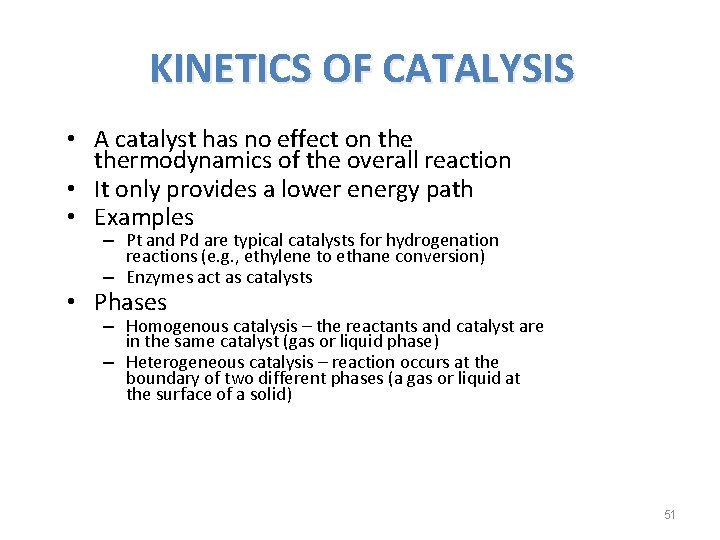
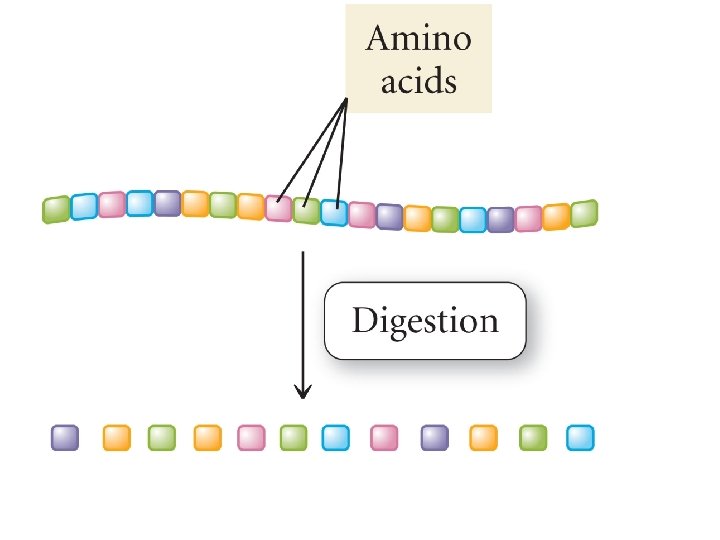
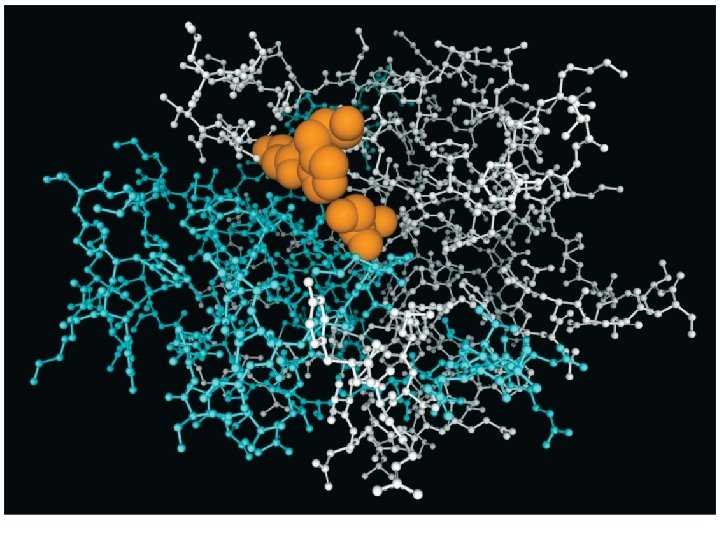
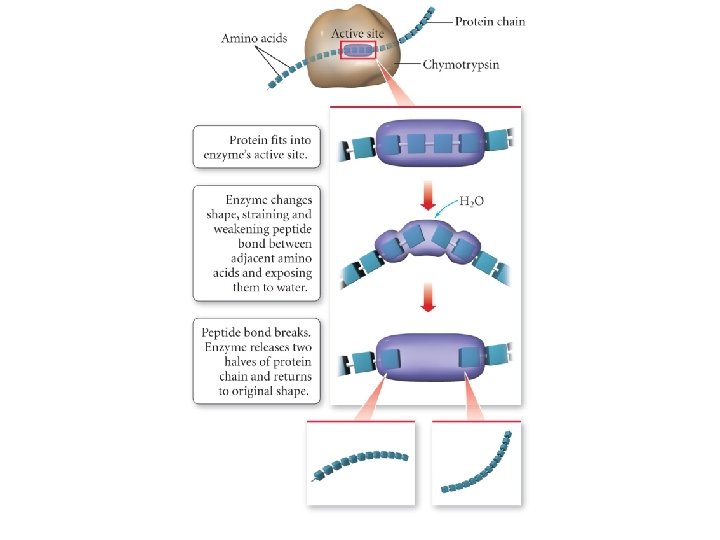
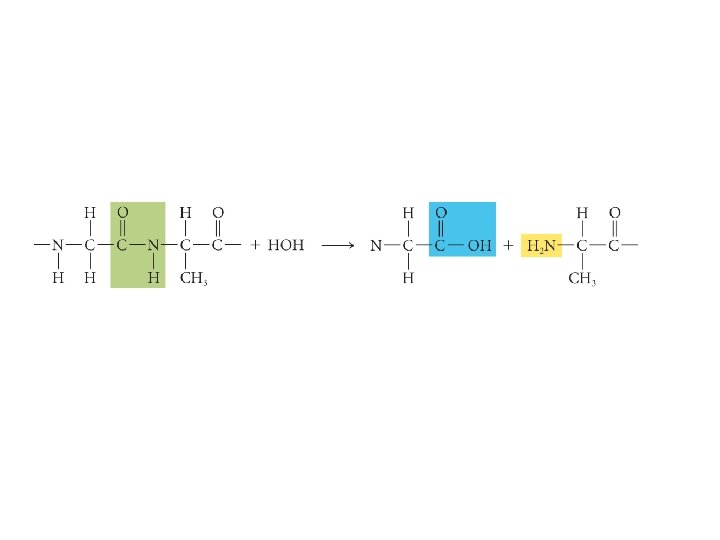
KINETICS OF CATALYSIS • A catalyst has no effect on thermodynamics of the overall reaction • It only provides a lower energy path • Examples – Pt and Pd are typical catalysts for hydrogenation reactions (e. g. , ethylene to ethane conversion) – Enzymes act as catalysts • Phases – Homogenous catalysis – the reactants and catalyst are in the same catalyst (gas or liquid phase) – Heterogeneous catalysis – reaction occurs at the boundary of two different phases (a gas or liquid at the surface of a solid) 51

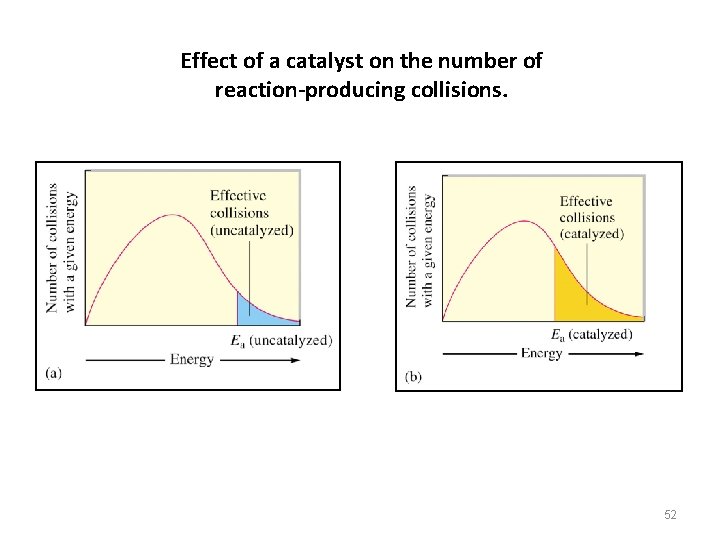
Effect of a catalyst on the number of reaction-producing collisions. 52







