Chemical Kinetics and Rates of Reaction Mc GrawHill


![A B time D[A] rate = Dt D[B] rate = Dt A B time D[A] rate = Dt D[B] rate = Dt](https://slidetodoc.com/presentation_image/b164df16f532566b6020cf85bcf6cd99/image-5.jpg)














- Slides: 45

Chemical Kinetics and Rates of Reaction Mc. Graw-Hill Chemistry Text Read Pg: 462 -482

Thermodynamics – does a reaction take place? Kinetics – how fast does a reaction proceed?

Chemical Kinetics • The study of rates of chemical reactions and the mechanisms (or steps) by which a chemical reaction takes place. • Reaction rates vary greatly – some are very fast (burning) and some are very slow (disintegration of a plastic bottle in sunlight).

• Rate of reaction: the change in concentration of a reactant or product per unit of time. • Rxn Rate (avg) = Δ [reactant or product] Δ time • Note: [square brackets] = mol/L
![A B time DA rate Dt DB rate Dt A B time D[A] rate = Dt D[B] rate = Dt](https://slidetodoc.com/presentation_image/b164df16f532566b6020cf85bcf6cd99/image-5.jpg)
A B time D[A] rate = Dt D[B] rate = Dt

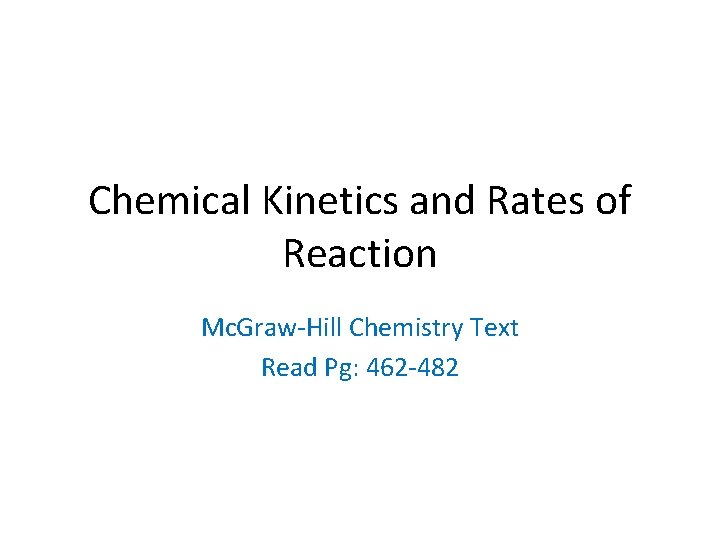
Br 2 (aq) + HCOOH (aq) 2 Br- (aq) + 2 H+ (aq) + CO 2 (g) time 393 nm light Detector D[Br 2] a DAbsorption

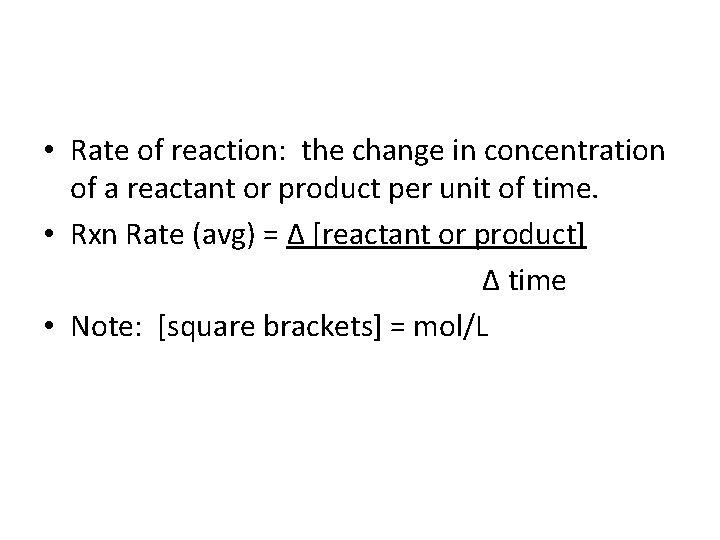
Br 2 (aq) + HCOOH (aq) 2 Br- (aq) + 2 H+ (aq) + CO 2 (g) slope of tangent [Br 2]final – [Br 2]initial D[Br 2] average rate = =Dt tfinal - tinitial instantaneous rate = rate for specific instance in time 13. 1

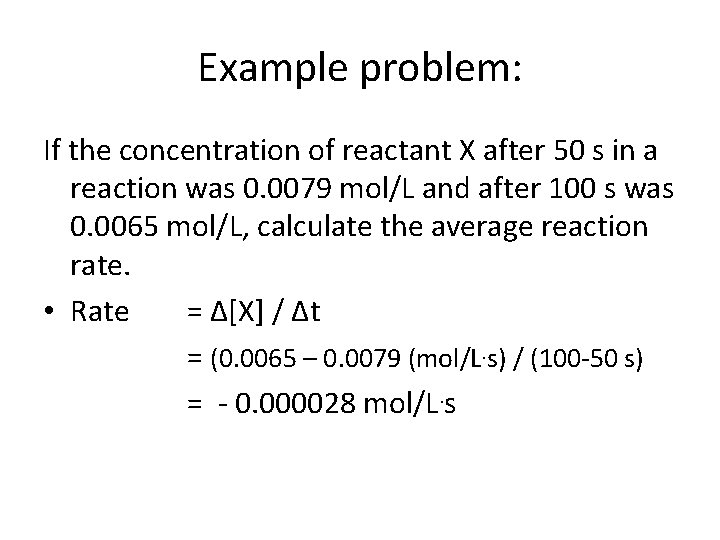
Example problem: If the concentration of reactant X after 50 s in a reaction was 0. 0079 mol/L and after 100 s was 0. 0065 mol/L, calculate the average reaction rate. • Rate = Δ[X] / Δt = (0. 0065 – 0. 0079 (mol/L. s) / (100 -50 s) = - 0. 000028 mol/L. s

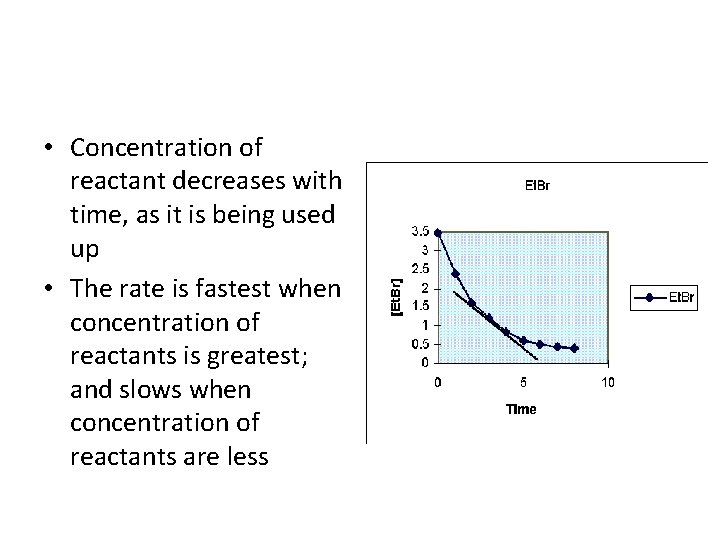
• Concentration of reactant decreases with time, as it is being used up • The rate is fastest when concentration of reactants is greatest; and slows when concentration of reactants are less

• The reactants are being used up as the reaction takes place. • What’s happening to the concentration of products over time? • It is increasing.

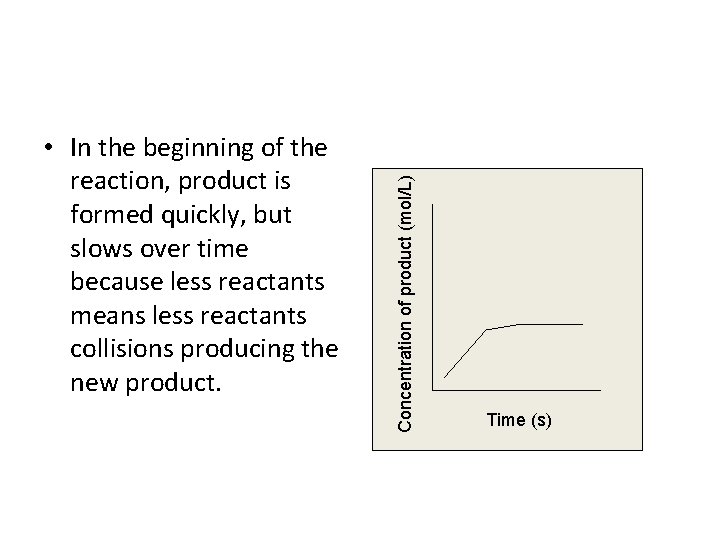
Concentration of product (mol/L) • In the beginning of the reaction, product is formed quickly, but slows over time because less reactants means less reactants collisions producing the new product. Time (s)

A chemical reaction… • In order for a chemical reaction to occur, the particles of the reactants must come in contact and collide with each other. • by calculating how many collisions are taking place per second and how quickly product is being produced, we learn that most collisions are not successful (no product formed)

• to think that reactant particles collide and products are automatically produced is over simplified. • There must be other requirements for a collision to be successful.

The Collision Theory • is an explanation of what is necessary for a chemical reaction to occur. • This theory states when a chemical reaction takes place, the reactant particles must meet two conditions (or requirements) during collision for the collisions to be successful

Conditions… 1. Particles must collide with a certain minimum amount of energy, called activation energy. • This energy is required to break chemical bonds in the reactants. • The energy of each particle is not important, it is the energy of the collision.

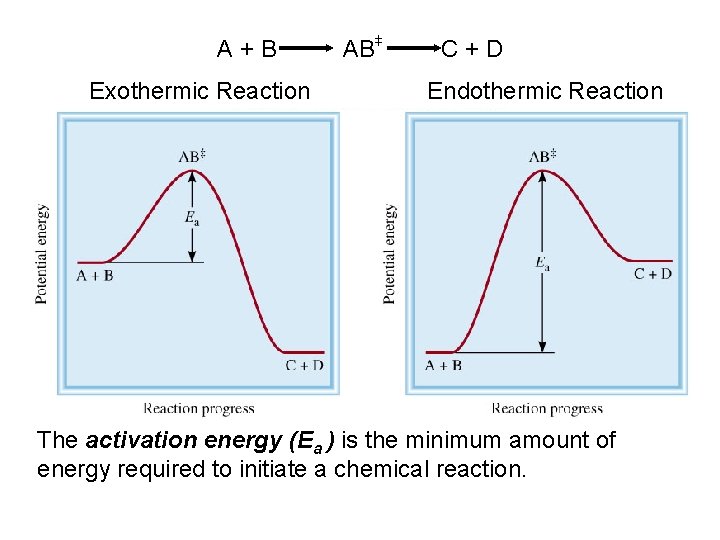
A+B Exothermic Reaction + + AB C+D Endothermic Reaction The activation energy (Ea ) is the minimum amount of energy required to initiate a chemical reaction.

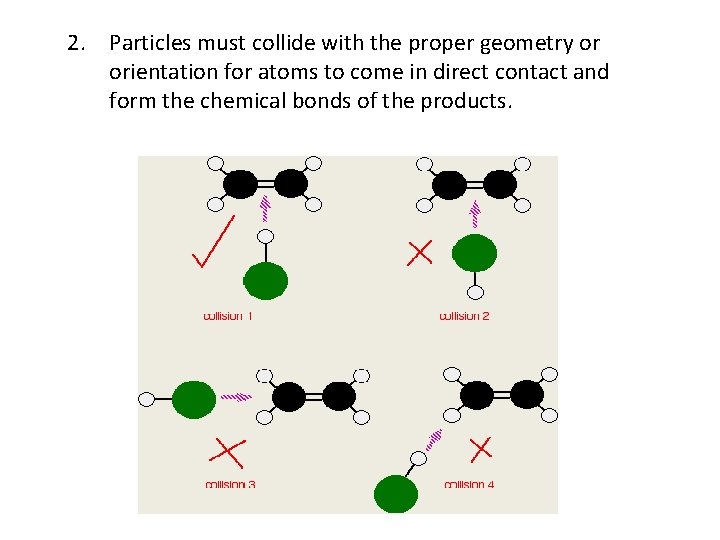
2. Particles must collide with the proper geometry or orientation for atoms to come in direct contact and form the chemical bonds of the products.

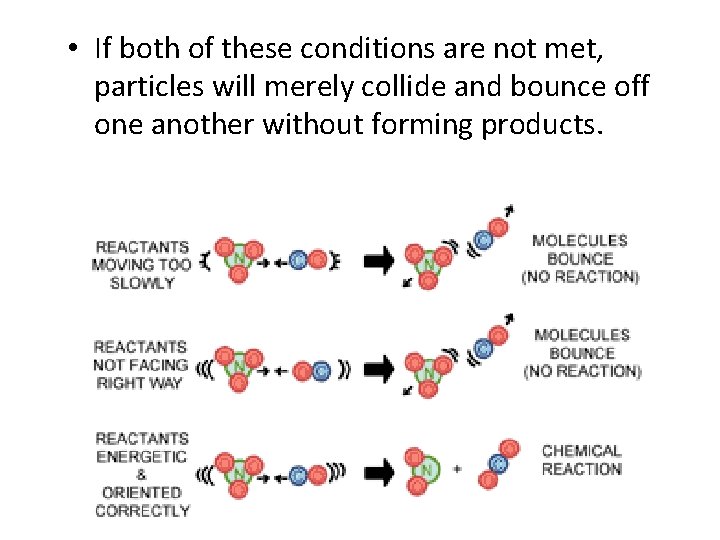
• If both of these conditions are not met, particles will merely collide and bounce off one another without forming products.

• Although, the percentage of successful collisions is extremely small, chemical reactions still take place at a reasonable rate because there are so many collisions per second between reactant particles.

According to collision theory, to increase the rate of reaction you therefore need. . . more frequent collisions increase particle speed or have more particles present more successful collisions give particles more energy or lower the activation energy

Factors that affect the Rate: • • • Concentration (and pressure) Temperature Amount of surface area Catalysts Nature of reactants

Concentration • A higher concentration of reactants leads to more effective collisions per unit time, which leads to an increasing reaction rate • We are not increasing the amount being made for a given balanced equation with limiting reactants, we are only speeding up how quickly those products are made.

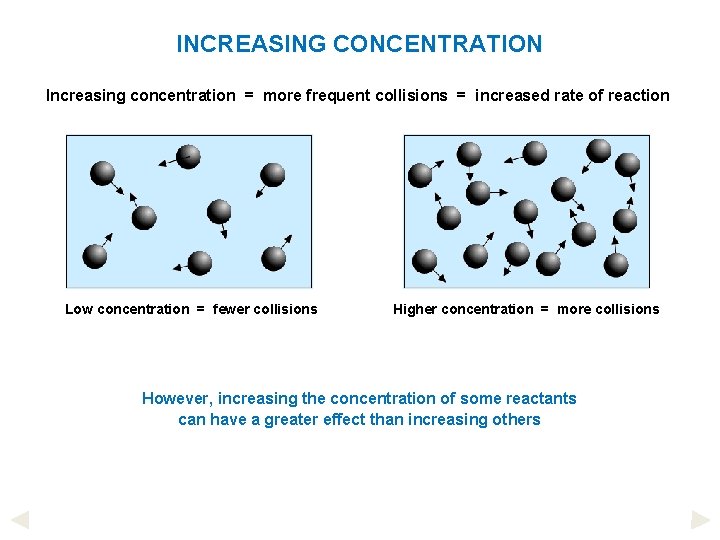
INCREASING CONCENTRATION Increasing concentration = more frequent collisions = increased rate of reaction Low concentration = fewer collisions Higher concentration = more collisions However, increasing the concentration of some reactants can have a greater effect than increasing others

Pressure • affects the rate of reaction, especially when you look at gases. • When you increase the pressure, the molecules have less space in which they can move. That greater concentration of molecules increases the number of collisions. • When you decrease the pressure, molecules don't hit each other as often. The lower pressure decreases the rate of reaction

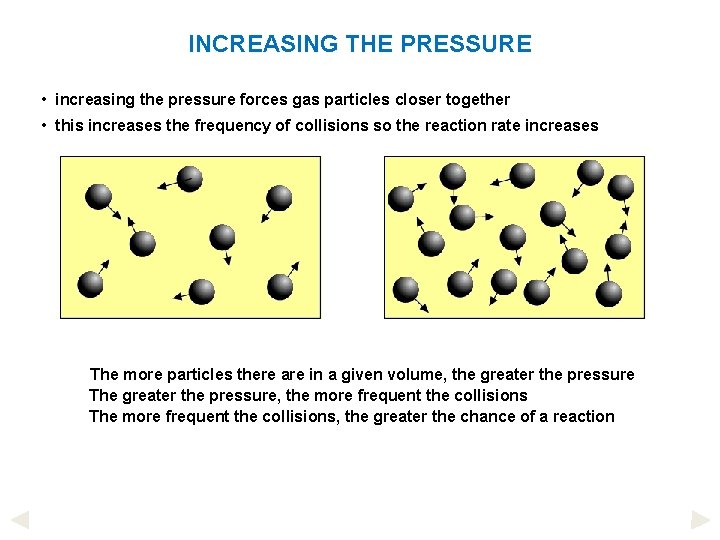
INCREASING THE PRESSURE • increasing the pressure forces gas particles closer together • this increases the frequency of collisions so the reaction rate increases The more particles there are in a given volume, the greater the pressure The greater the pressure, the more frequent the collisions The more frequent the collisions, the greater the chance of a reaction

Temperature • Temperature is a measure of the kinetic energy of a system, so higher temperature implies higher average kinetic energy of molecules and more collisions per unit time.

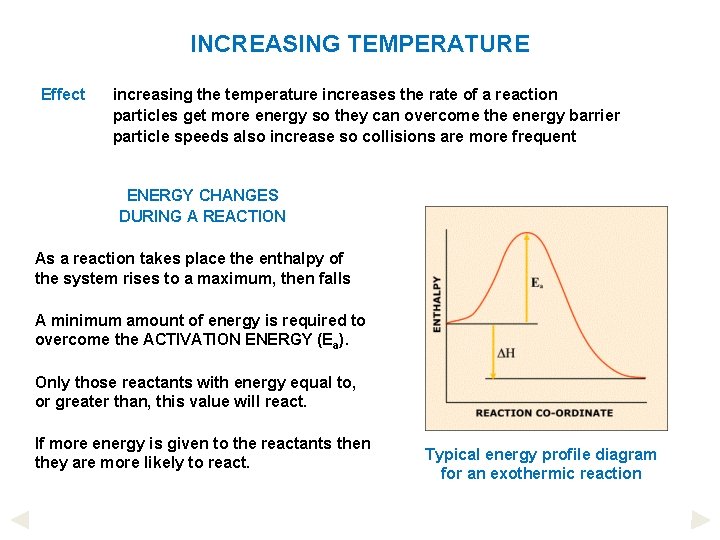
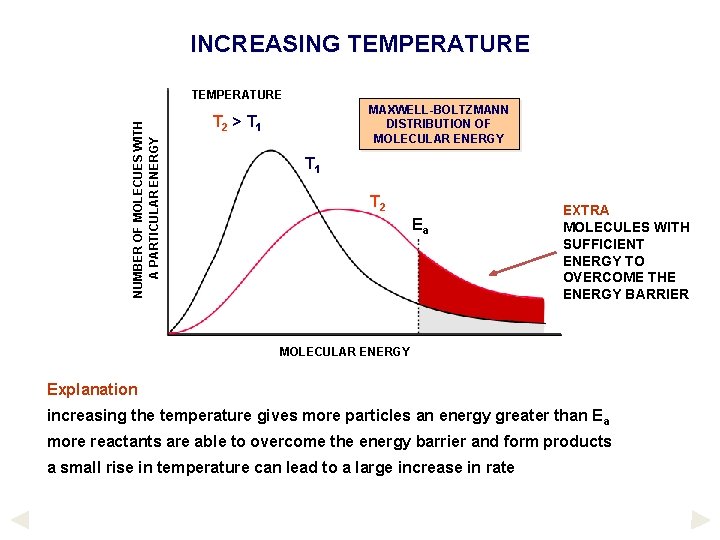
INCREASING TEMPERATURE Effect increasing the temperature increases the rate of a reaction particles get more energy so they can overcome the energy barrier particle speeds also increase so collisions are more frequent ENERGY CHANGES DURING A REACTION As a reaction takes place the enthalpy of the system rises to a maximum, then falls A minimum amount of energy is required to overcome the ACTIVATION ENERGY (Ea). Only those reactants with energy equal to, or greater than, this value will react. If more energy is given to the reactants then they are more likely to react. Typical energy profile diagram for an exothermic reaction

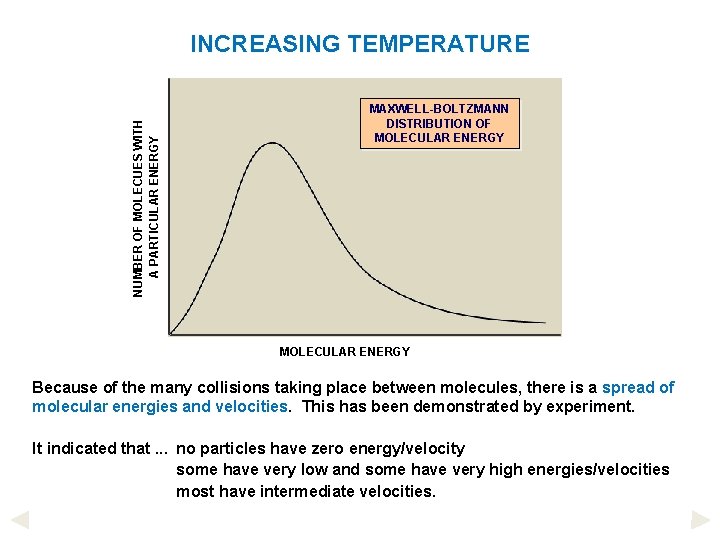
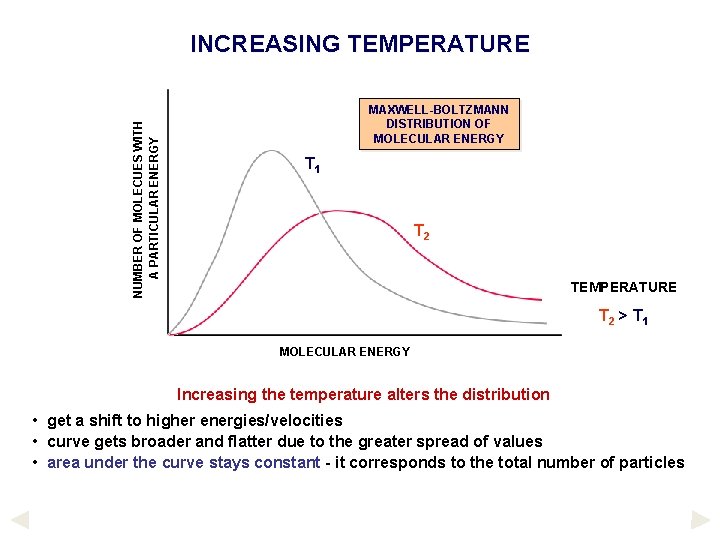
NUMBER OF MOLECUES WITH A PARTICULAR ENERGY INCREASING TEMPERATURE MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY Because of the many collisions taking place between molecules, there is a spread of molecular energies and velocities. This has been demonstrated by experiment. It indicated that. . . no particles have zero energy/velocity some have very low and some have very high energies/velocities most have intermediate velocities.

NUMBER OF MOLECUES WITH A PARTICULAR ENERGY INCREASING TEMPERATURE MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY T 1 T 2 TEMPERATURE T 2 > T 1 MOLECULAR ENERGY Increasing the temperature alters the distribution • get a shift to higher energies/velocities • curve gets broader and flatter due to the greater spread of values • area under the curve stays constant - it corresponds to the total number of particles

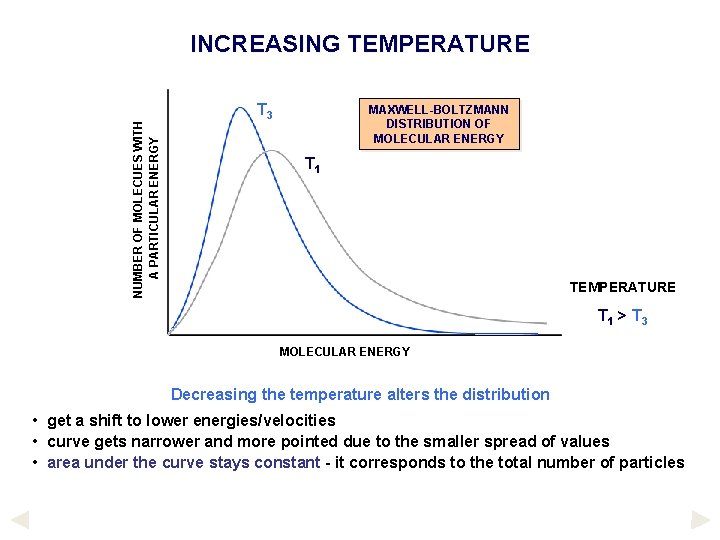
NUMBER OF MOLECUES WITH A PARTICULAR ENERGY INCREASING TEMPERATURE T 3 MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY T 1 TEMPERATURE T 1 > T 3 MOLECULAR ENERGY Decreasing the temperature alters the distribution • get a shift to lower energies/velocities • curve gets narrower and more pointed due to the smaller spread of values • area under the curve stays constant - it corresponds to the total number of particles

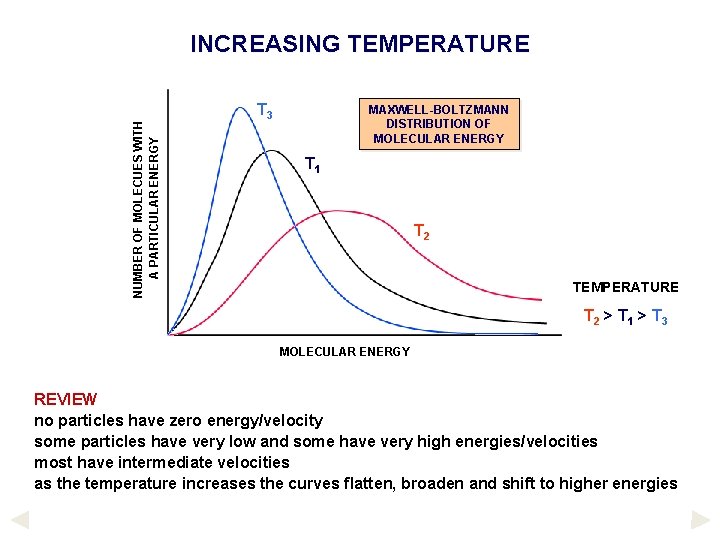
NUMBER OF MOLECUES WITH A PARTICULAR ENERGY INCREASING TEMPERATURE T 3 MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY T 1 T 2 TEMPERATURE T 2 > T 1 > T 3 MOLECULAR ENERGY REVIEW no particles have zero energy/velocity some particles have very low and some have very high energies/velocities most have intermediate velocities as the temperature increases the curves flatten, broaden and shift to higher energies

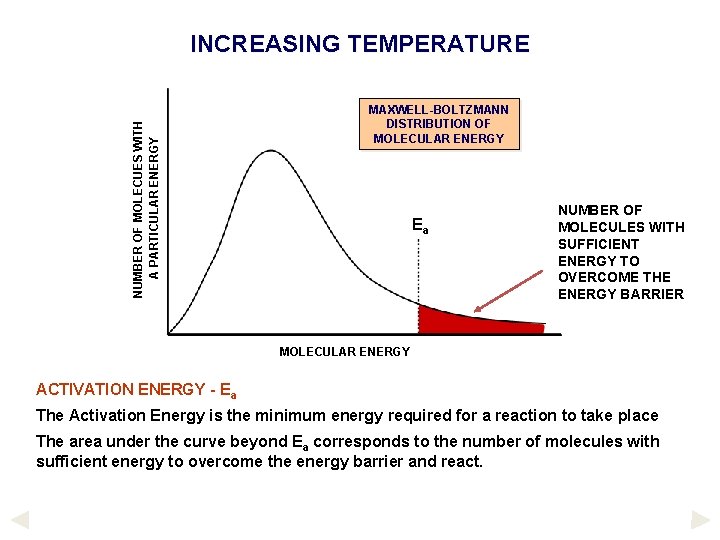
NUMBER OF MOLECUES WITH A PARTICULAR ENERGY INCREASING TEMPERATURE MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY Ea NUMBER OF MOLECULES WITH SUFFICIENT ENERGY TO OVERCOME THE ENERGY BARRIER MOLECULAR ENERGY ACTIVATION ENERGY - Ea The Activation Energy is the minimum energy required for a reaction to take place The area under the curve beyond Ea corresponds to the number of molecules with sufficient energy to overcome the energy barrier and react.

INCREASING TEMPERATURE NUMBER OF MOLECUES WITH A PARTICULAR ENERGY TEMPERATURE MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY T 2 > T 1 T 2 Ea EXTRA MOLECULES WITH SUFFICIENT ENERGY TO OVERCOME THE ENERGY BARRIER MOLECULAR ENERGY Explanation increasing the temperature gives more particles an energy greater than E a more reactants are able to overcome the energy barrier and form products a small rise in temperature can lead to a large increase in rate

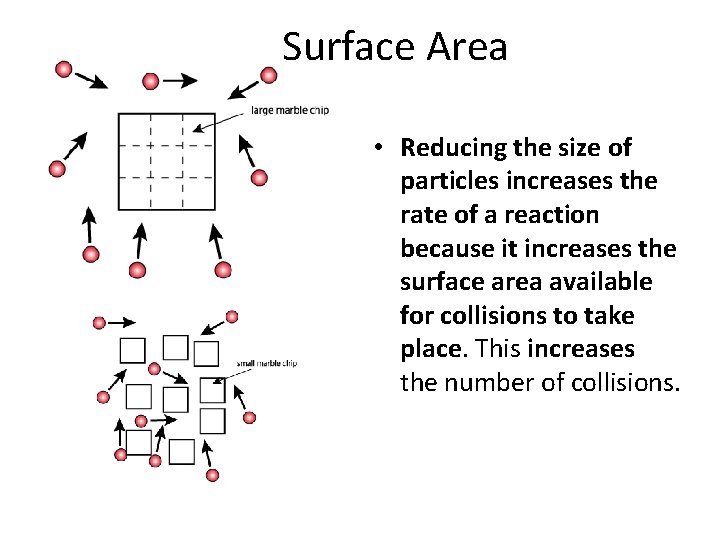
Surface Area • Reducing the size of particles increases the rate of a reaction because it increases the surface area available for collisions to take place. This increases the number of collisions.

Catalysts • A catalyst is a substance that speeds up a reaction without being used up itself. • Some reactions have catalysts that can speed them up, but for many reactions there is no catalyst that works. • How do they work?

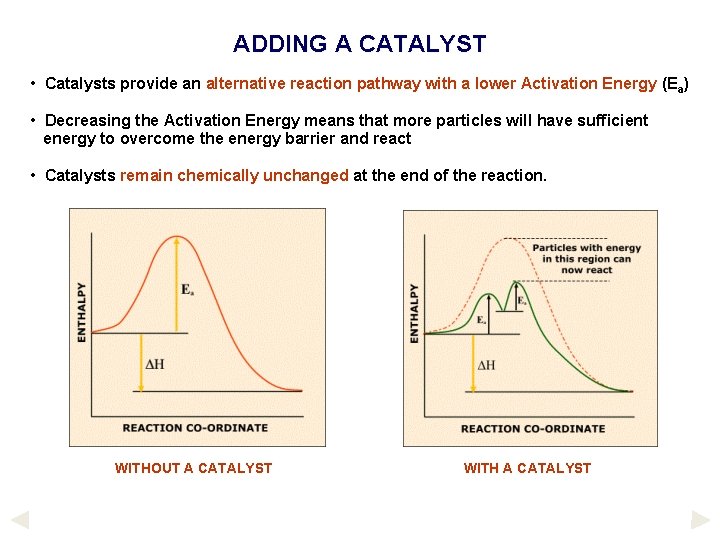
ADDING A CATALYST • Catalysts provide an alternative reaction pathway with a lower Activation Energy (Ea) • Decreasing the Activation Energy means that more particles will have sufficient energy to overcome the energy barrier and react • Catalysts remain chemically unchanged at the end of the reaction. WITHOUT A CATALYST WITH A CATALYST

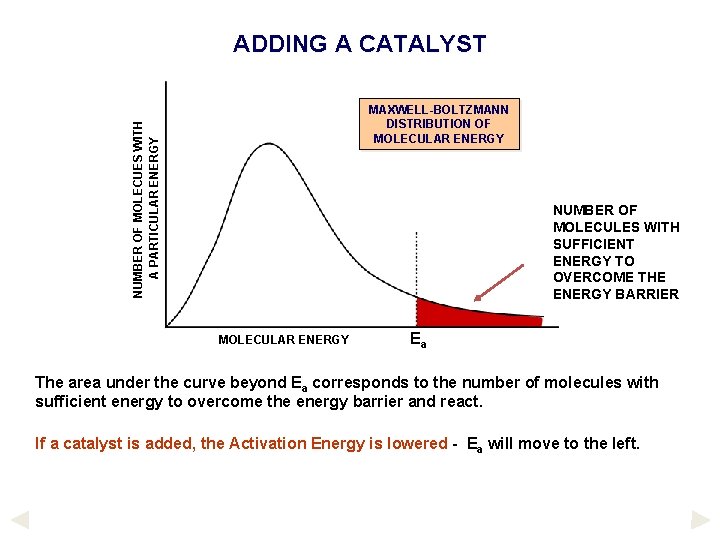
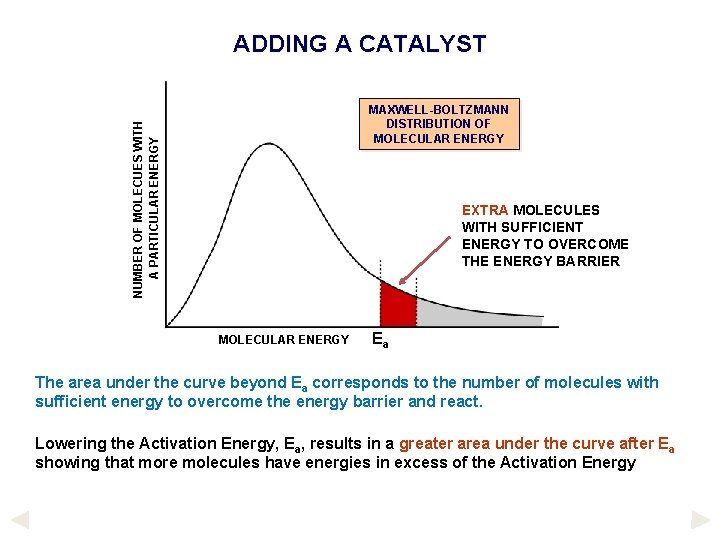
ADDING A CATALYST NUMBER OF MOLECUES WITH A PARTICULAR ENERGY MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY NUMBER OF MOLECULES WITH SUFFICIENT ENERGY TO OVERCOME THE ENERGY BARRIER MOLECULAR ENERGY Ea The area under the curve beyond Ea corresponds to the number of molecules with sufficient energy to overcome the energy barrier and react. If a catalyst is added, the Activation Energy is lowered - Ea will move to the left.

ADDING A CATALYST NUMBER OF MOLECUES WITH A PARTICULAR ENERGY MAXWELL-BOLTZMANN DISTRIBUTION OF MOLECULAR ENERGY EXTRA MOLECULES WITH SUFFICIENT ENERGY TO OVERCOME THE ENERGY BARRIER MOLECULAR ENERGY Ea The area under the curve beyond Ea corresponds to the number of molecules with sufficient energy to overcome the energy barrier and react. Lowering the Activation Energy, Ea, results in a greater area under the curve after Ea showing that more molecules have energies in excess of the Activation Energy

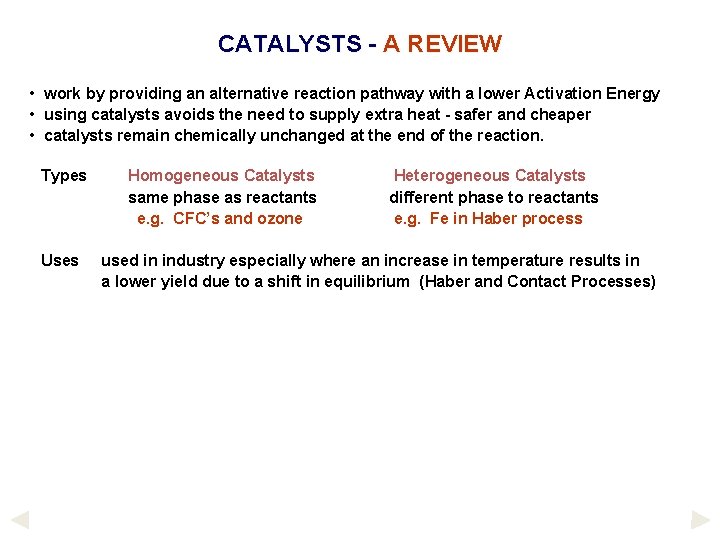
CATALYSTS - A REVIEW • work by providing an alternative reaction pathway with a lower Activation Energy • using catalysts avoids the need to supply extra heat - safer and cheaper • catalysts remain chemically unchanged at the end of the reaction. Types Uses Homogeneous Catalysts same phase as reactants e. g. CFC’s and ozone Heterogeneous Catalysts different phase to reactants e. g. Fe in Haber process used in industry especially where an increase in temperature results in a lower yield due to a shift in equilibrium (Haber and Contact Processes)

Nature of Reactants • Reactants with large number of chemical bonds that have to be broken, or with strong chemical bonds that have to be broken, will lead to a slower rate. • The strong bonds will cause a high activation energy, leading fewer collisions being successful.

• More complex reactant molecules with more atoms, will have more difficulty lining up properly to have a successful reaction. This will also lead to a lower reaction rate.

Good rule of thumb… – Ions will react more quickly, because already broken up. – Simple molecules (non-metals covalently bonded) will take more time – Complex molecules and ionic compounds (metal and nonmetal bonded) will take the most time because the strong ionic bonds need lots of energy to be broken, and the complex molecules will be hard to have proper geometry (line up)

Practice Place the following reactions from fastest to slowest, based on the previous slides. 1. 2 Cu+2 + 2 I- 2 Cu+ + I 2 2. 2 Na. Cl 2 Na + Cl 2 3. Cl 2 2 Cl Ans: 1 is fastest, then 3, and 2 is slowest

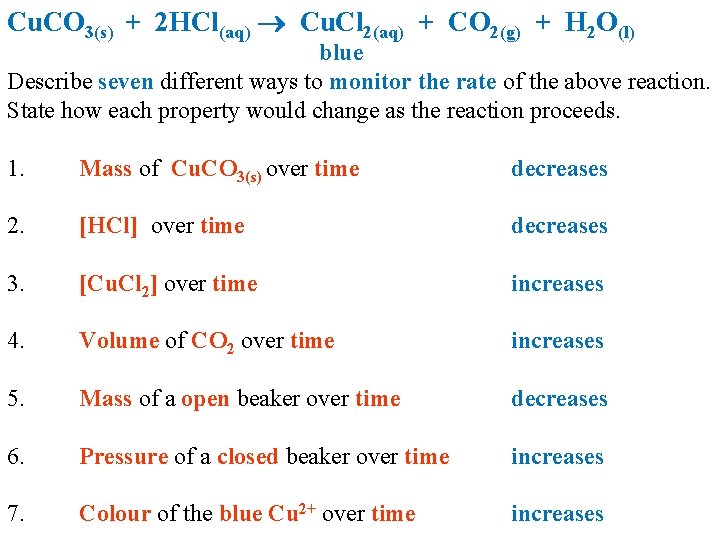
Cu. CO 3(s) + 2 HCl(aq) Cu. Cl 2(aq) + CO 2(g) + H 2 O(l) blue Describe seven different ways to monitor the rate of the above reaction. State how each property would change as the reaction proceeds. 1. Mass of Cu. CO 3(s) over time decreases 2. [HCl] over time decreases 3. [Cu. Cl 2] over time increases 4. Volume of CO 2 over time increases 5. Mass of a open beaker over time decreases 6. Pressure of a closed beaker over time increases 7. Colour of the blue Cu 2+ over time increases

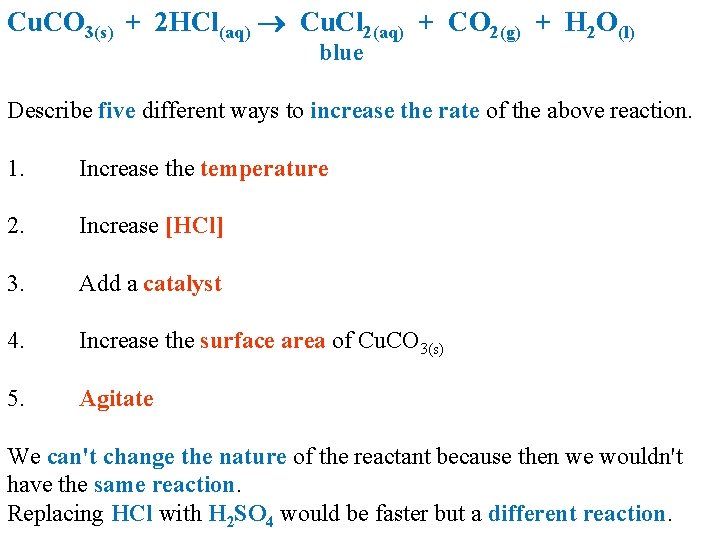
Cu. CO 3(s) + 2 HCl(aq) Cu. Cl 2(aq) + CO 2(g) + H 2 O(l) blue Describe five different ways to increase the rate of the above reaction. 1. Increase the temperature 2. Increase [HCl] 3. Add a catalyst 4. Increase the surface area of Cu. CO 3(s) 5. Agitate We can't change the nature of the reactant because then we wouldn't have the same reaction. Replacing HCl with H 2 SO 4 would be faster but a different reaction.