Chemical Foundations Notes Formulas of Compounds 1 Symbols
Chemical Foundations Notes
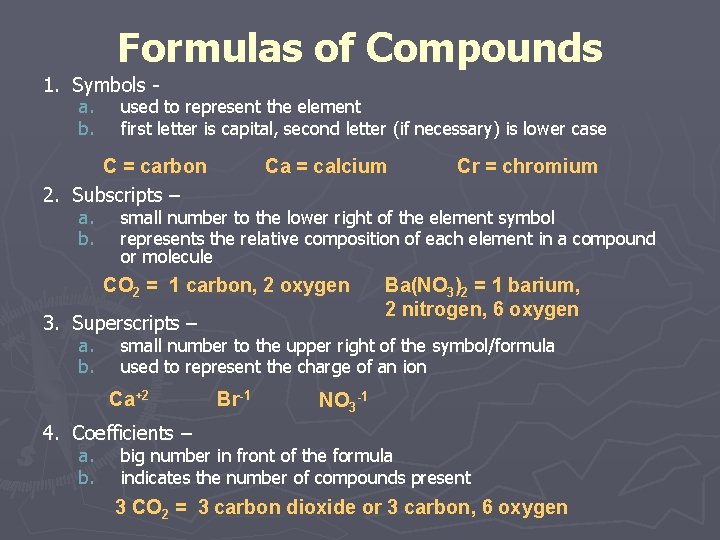
Formulas of Compounds 1. Symbols a. b. used to represent the element first letter is capital, second letter (if necessary) is lower case C = carbon 2. Subscripts – a. b. Ca = calcium small number to the lower right of the element symbol represents the relative composition of each element in a compound or molecule CO 2 = 1 carbon, 2 oxygen 3. Superscripts – a. b. Cr = chromium Ba(NO 3)2 = 1 barium, 2 nitrogen, 6 oxygen small number to the upper right of the symbol/formula used to represent the charge of an ion Ca+2 Br-1 NO 3 -1 4. Coefficients – a. b. big number in front of the formula indicates the number of compounds present 3 CO 2 = 3 carbon dioxide or 3 carbon, 6 oxygen
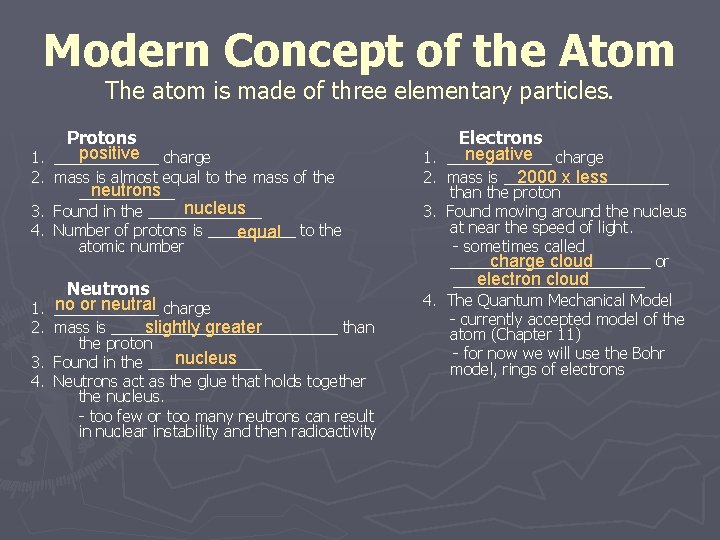
Modern Concept of the Atom The atom is made of three elementary particles. Protons positive charge 1. ______ 2. mass is almost equal to the mass of the neutrons ______ nucleus 3. Found in the _______ 4. Number of protons is _____ equal to the atomic number Neutrons no or neutral charge 1. ______ slightly greater 2. mass is _____________ than the proton nucleus 3. Found in the _______ 4. Neutrons act as the glue that holds together the nucleus. - too few or too many neutrons can result in nuclear instability and then radioactivity Electrons negative charge 1. ______ 2. mass is __________ 2000 x less than the proton 3. Found moving around the nucleus at near the speed of light. - sometimes called charge cloud ____________ or electron cloud ___________ 4. The Quantum Mechanical Model - currently accepted model of the atom (Chapter 11) - for now we will use the Bohr model, rings of electrons
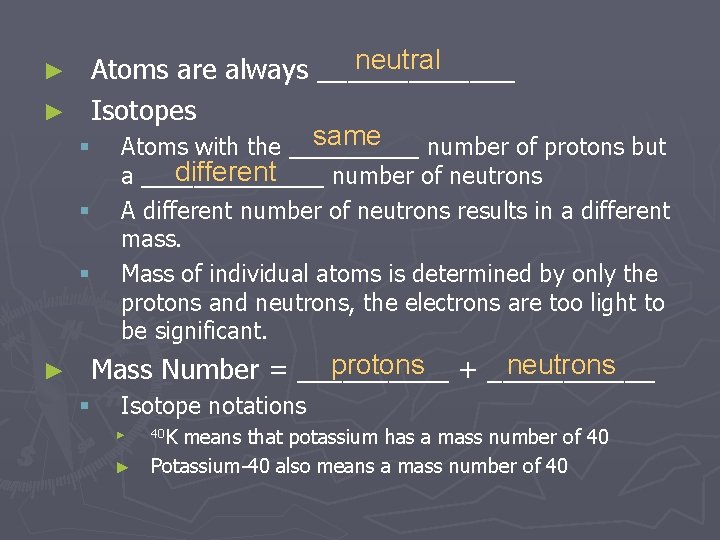
neutral Atoms are always _______ ► Isotopes same § Atoms with the _____ number of protons but different a _______ number of neutrons ► § § ► A different number of neutrons results in a different mass. Mass of individual atoms is determined by only the protons and neutrons, the electrons are too light to be significant. protons + ______ neutrons Mass Number = _____ § Isotope notations means that potassium has a mass number of 40 ► Potassium-40 also means a mass number of 40 ► 40 K
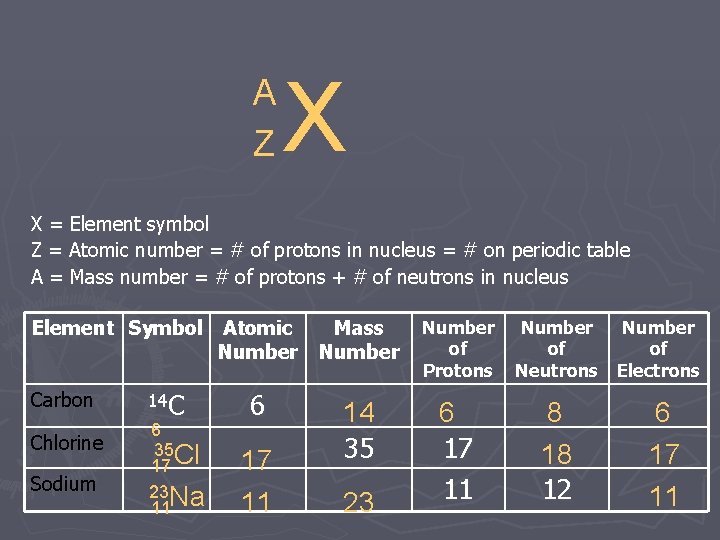
A Z X X = Element symbol Z = Atomic number = # of protons in nucleus = # on periodic table A = Mass number = # of protons + # of neutrons in nucleus Element Symbol Atomic Mass Number Carbon Chlorine Sodium 14 C 6 35 Cl 17 23 Na 11 6 17 11 14 35 23 Number of Protons 6 17 11 Number of of Neutrons Electrons 8 18 12 6 17 11
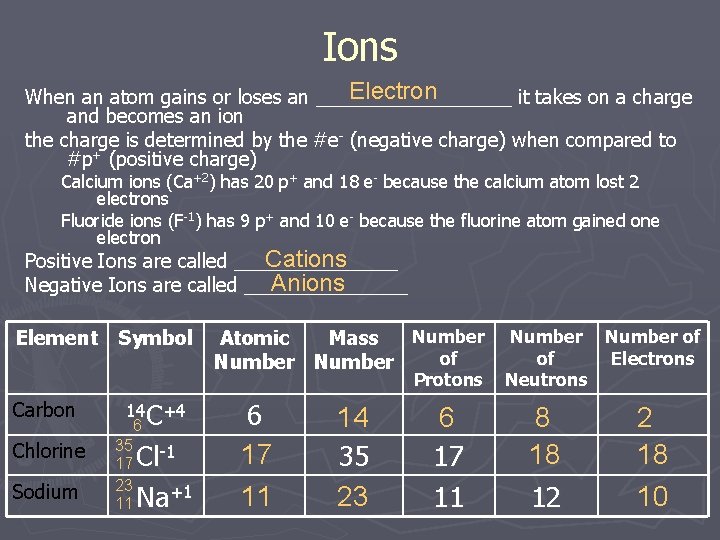
Ions Electron When an atom gains or loses an _________ it takes on a charge and becomes an ion the charge is determined by the #e- (negative charge) when compared to #p+ (positive charge) Calcium ions (Ca+2) has 20 p+ and 18 e- because the calcium atom lost 2 electrons Fluoride ions (F-1) has 9 p+ and 10 e- because the fluorine atom gained one electron Cations Positive Ions are called ________ Anions Negative Ions are called ________ Element Symbol Number Atomic Mass of Number Protons Carbon Chlorine Sodium 14 C+4 6 35 17 23 11 Cl-1 Na+1 6 17 11 14 35 23 6 17 11 Number of of Electrons Neutrons 8 18 12 2 18 10
- Slides: 6