Chemical formulation Metallic hydrides Formula Stock name Systematic

- Slides: 6

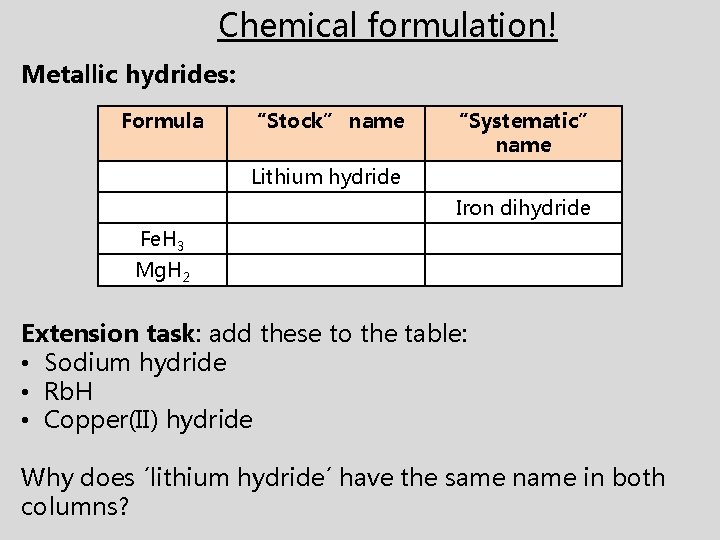
Chemical formulation! Metallic hydrides: Formula “Stock” name “Systematic” name Lithium hydride Iron dihydride Fe. H 3 Mg. H 2 Extension task: add these to the table: • Sodium hydride • Rb. H • Copper(II) hydride Why does ´lithium hydride´ have the same name in both columns?

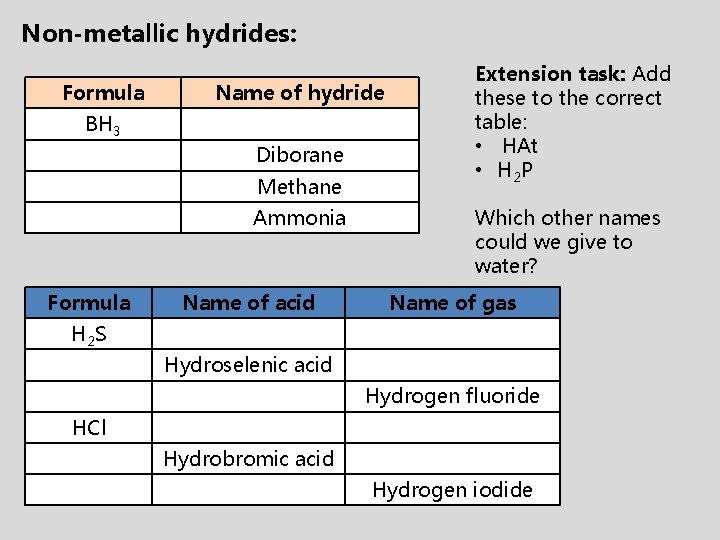
Non-metallic hydrides: Formula Name of hydride BH 3 Diborane Methane Ammonia Formula Name of acid Extension task: Add these to the correct table: • HAt • H 2 P Which other names could we give to water? Name of gas H 2 S Hydroselenic acid Hydrogen fluoride HCl Hydrobromic acid Hydrogen iodide

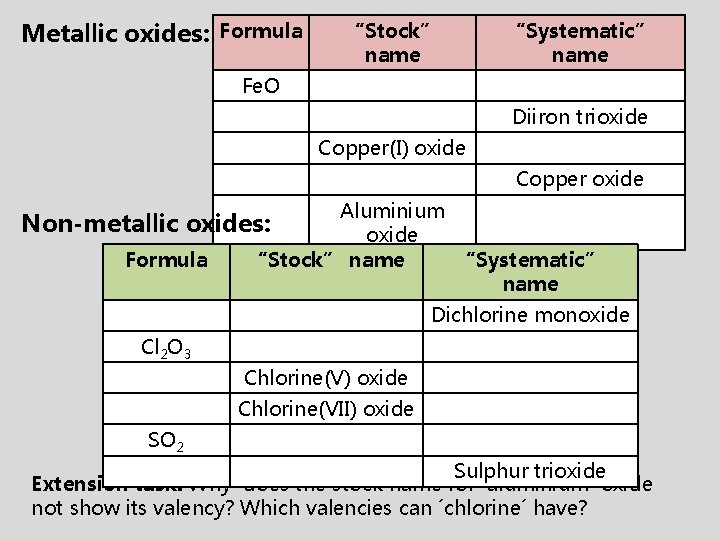
Metallic oxides: Formula “Stock” name “Systematic” name Fe. O Diiron trioxide Copper(I) oxide Copper oxide Aluminium oxide “Stock” name “Systematic” name Non-metallic oxides: Formula Dichlorine monoxide Cl 2 O 3 Chlorine(V) oxide Chlorine(VII) oxide SO 2 Sulphur trioxide Extension task: Why does the stock name for ´aluminium´ oxide not show its valency? Which valencies can ´chlorine´ have?

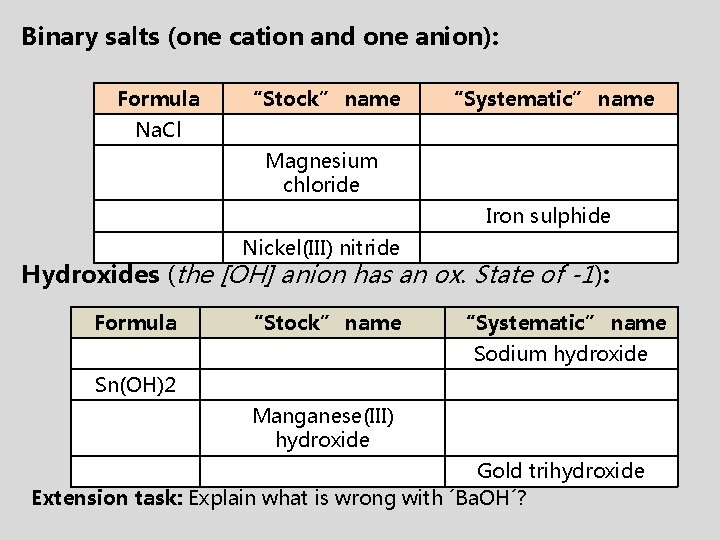
Binary salts (one cation and one anion): Formula “Stock” name “Systematic” name Na. Cl Magnesium chloride Iron sulphide Nickel(III) nitride Hydroxides (the [OH] anion has an ox. State of -1): Formula “Stock” name “Systematic” name Sodium hydroxide Sn(OH)2 Manganese(III) hydroxide Gold trihydroxide Extension task: Explain what is wrong with ´Ba. OH´?

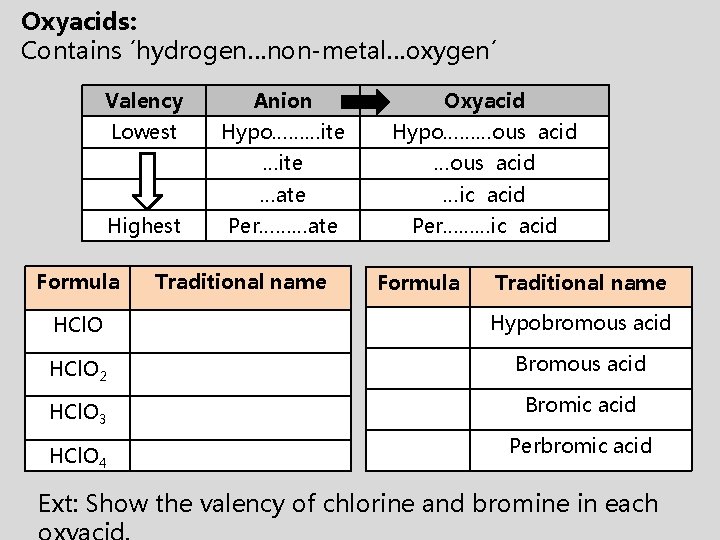
Oxyacids: Contains ´hydrogen…non-metal…oxygen´ Valency Anion Oxyacid Lowest Hypo………ite Hypo………ous acid …ite …ous acid …ate …ic acid Per………ate Per………ic acid Highest Formula Traditional name HCl. O Hypobromous acid HCl. O 2 Bromous acid HCl. O 3 Bromic acid HCl. O 4 Perbromic acid Ext: Show the valency of chlorine and bromine in each

Practice questions 1. Sr. O 2. Hg 2 CO 3 3. NO 2 4. BH 3 5. Cu. OH 6. Pd. H 2 7. HIO 8. K 2 Mn. O 4 9. KIO 4 10. HBr. O 3 11. Co 2 O 3 12. (NH 4)3 PO 4 1. Mercury(II) sulfide 2. Calcium carbonate 3. Zinc dichromate 4. Ammonia 5. Nitric acid 6. Sulfuric acid 7. Phosphorus(III) iodide 8. Copper(II) hydroxide 9. lead(II) iodate 10. Iron(III) fluoride 11. Ammonium iodide 12. Sodium hypochlorite 13. Zinc chromate 14. Potassium peroxide