Chemical Formulas The grammar of Chemistry Elements form





- Slides: 20

Chemical Formulas: The grammar of Chemistry Elements form two major types of bonds: • Ionic • Covalent The type of bonding determines the way compounds are named. In addition, there are some special names for compounds which are organic or acids. • In this chapter, we will learn how to identify the correct system to name compounds, and learn how the names of compounds help us learn about the properties of substances and their composition.

Names are very revealing: you can make some assumptions about people just by knowing their name! • Lets take the • Jose is a name • Rover is a name: for a male person. • Jose Garcia – animal. describe some • The last name • Probably, of the traits of indicates that Rover likes to this person. Jose is a person, wander. not an animal. • Rover • He probably is a dog, because Latino. Rover is a • … common name for a dog. • …

Names of Elements • Many elements are in honor of scientists. • Many elements honor the historical usage or discovery of the element. • Some elements are named systematically (typically man-made elements. • Many elements have the endings, -ium, -ine, –ogen or -on • As a general rule, -ium endings are on metals, -ine, -ogen, and -on endings are on nonmetals, Lithium Sodium Potassium Cesium Carbon, Nitrogen Chlorine

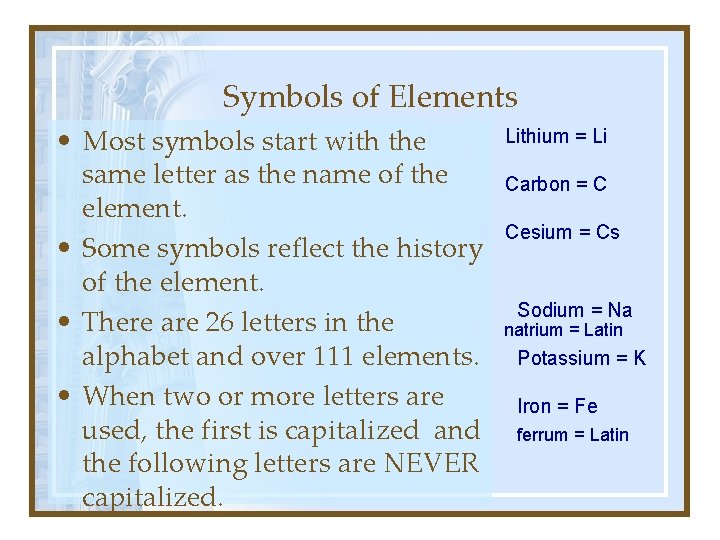
Symbols of Elements • Most symbols start with the same letter as the name of the element. • Some symbols reflect the history of the element. • There are 26 letters in the alphabet and over 111 elements. • When two or more letters are used, the first is capitalized and the following letters are NEVER capitalized. Lithium = Li Carbon = C Cesium = Cs Sodium = Na natrium = Latin Potassium = K Iron = Fe ferrum = Latin

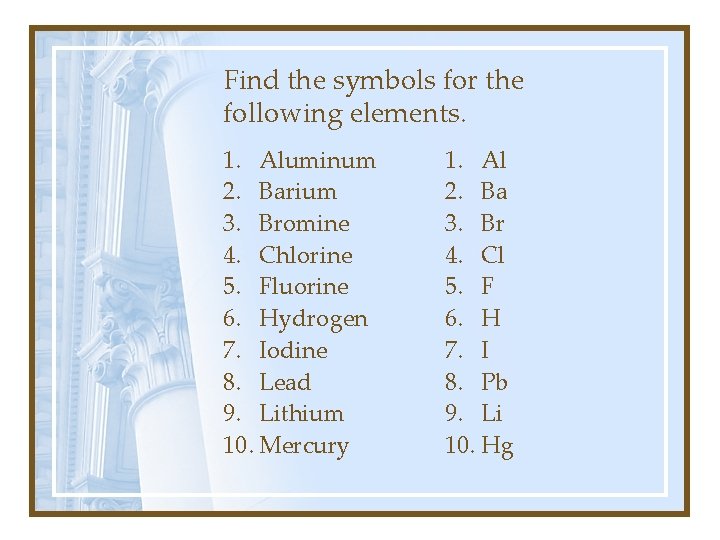
Find the symbols for the following elements. 1. Aluminum 2. Barium 3. Bromine 4. Chlorine 5. Fluorine 6. Hydrogen 7. Iodine 8. Lead 9. Lithium 10. Mercury 1. Al 2. Ba 3. Br 4. Cl 5. F 6. H 7. I 8. Pb 9. Li 10. Hg

end day 1 Find the Names for the following elements. 1. Ne 2. O 3. Ni 4. P 5. K 6. Na 7. Cl 8. S 9. Sn 10. Hg 1. neon 2. oxygen 3. nickel 4. phosphorous 5. potassium 6. sodium 7. chlorine 8. sulfur 9. tin 10. mercury

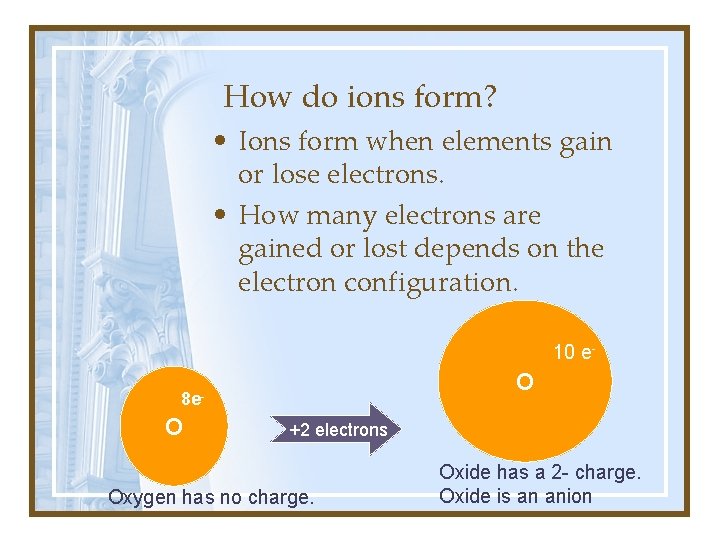
How do ions form? • Ions form when elements gain or lose electrons. • How many electrons are gained or lost depends on the electron configuration. 10 e- O 8 e- O +2 electrons Oxygen has no charge. Oxide has a 2 - charge. Oxide is an anion

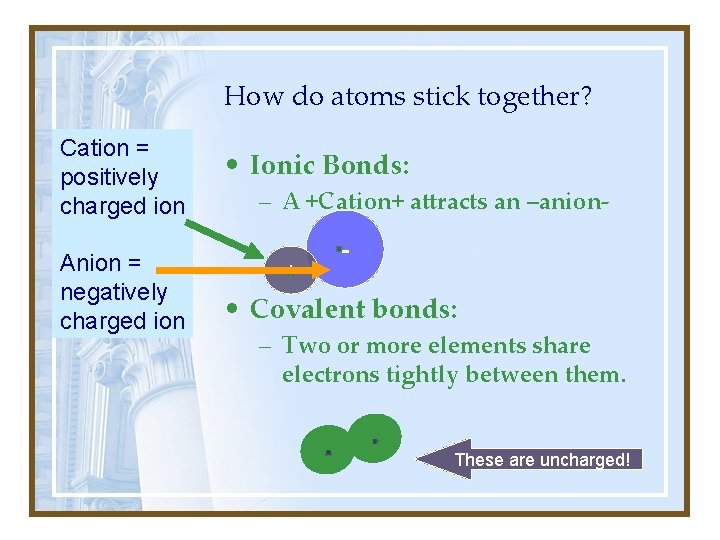
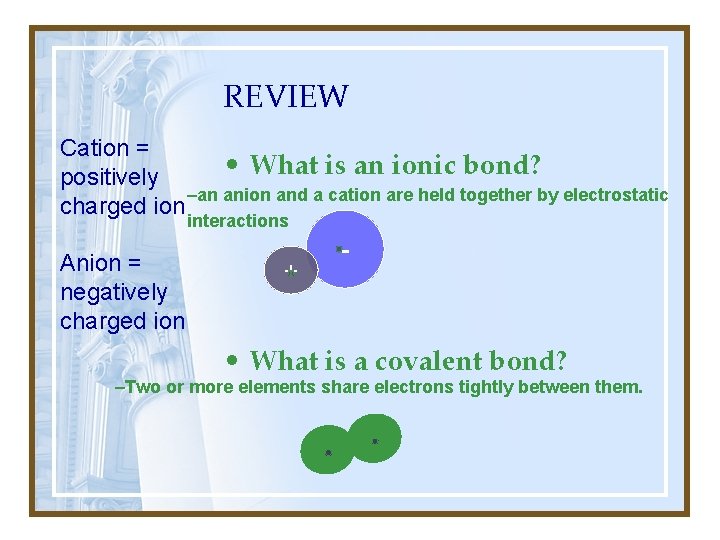
How do atoms stick together? Cation = positively charged ion Anion = negatively charged ion • Ionic Bonds: – A +Cation+ attracts an –anion+ - • Covalent bonds: – Two or more elements share electrons tightly between them. These are uncharged!

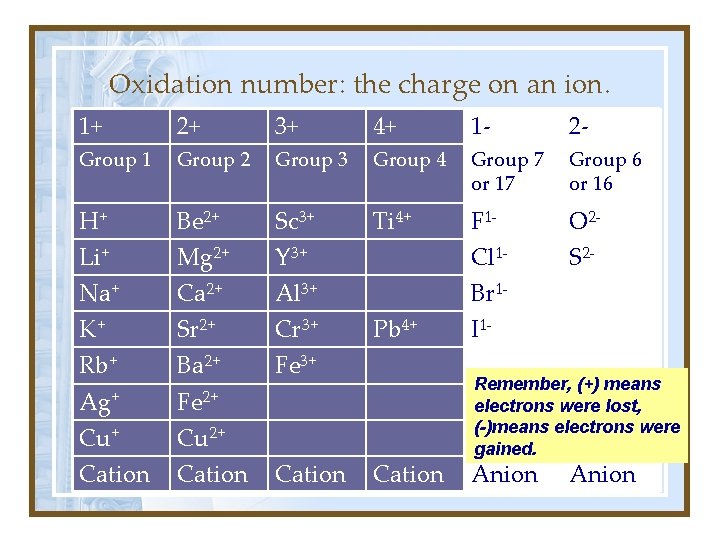
Oxidation number: the charge on an ion. 1+ 2+ 3+ 4+ 1 - 2 - Group 1 Group 2 Group 3 Group 4 Group 7 or 17 Group 6 or 16 H+ Be 2+ Sc 3+ Ti 4+ F 1 - O 2 - Li+ Mg 2+ Y 3+ Cl 1 - S 2 - Na+ Ca 2+ Al 3+ Br 1 - K+ Sr 2+ Cr 3+ Rb+ Ba 2+ Fe 3+ Ag+ Fe 2+ Cu 2+ Cation Pb 4+ Cation I 1 Remember, (+) means electrons were lost, (-)means electrons were gained. Anion

What is an ion? • An ion is an atom which has gained or lost electrons, and therefore – carries a charge. What is a polyatomic ion? • A polyatomic ion consists of more than one element covalently bonded together, which collectively carries a charge.

REVIEW Cation = • What is an ionic bond? positively –an anion and a cation are held together by electrostatic charged ion interactions Anion = negatively charged ion + - • What is a covalent bond? –Two or more elements share electrons tightly between them.

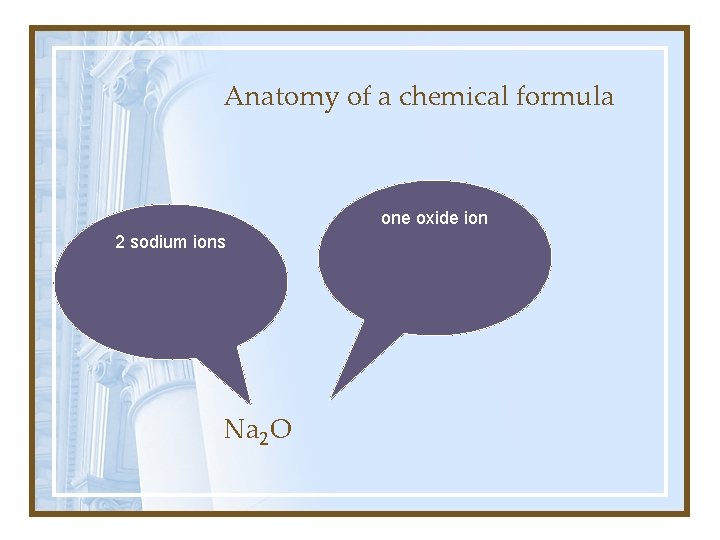
Anatomy of a chemical formula one oxide ion 2 sodium ions Na 2 O

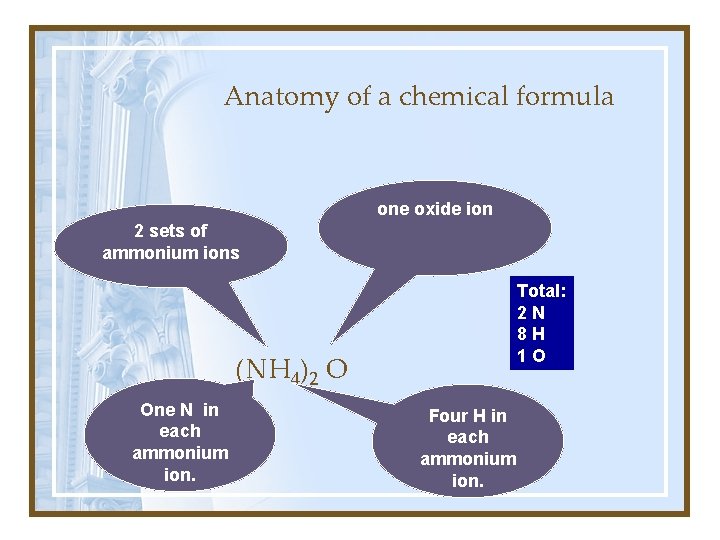
Anatomy of a chemical formula one oxide ion 2 sets of ammonium ions Total: 2 N 8 H 1 O (NH 4)2 O One N in each ammonium ion. Four H in each ammonium ion.

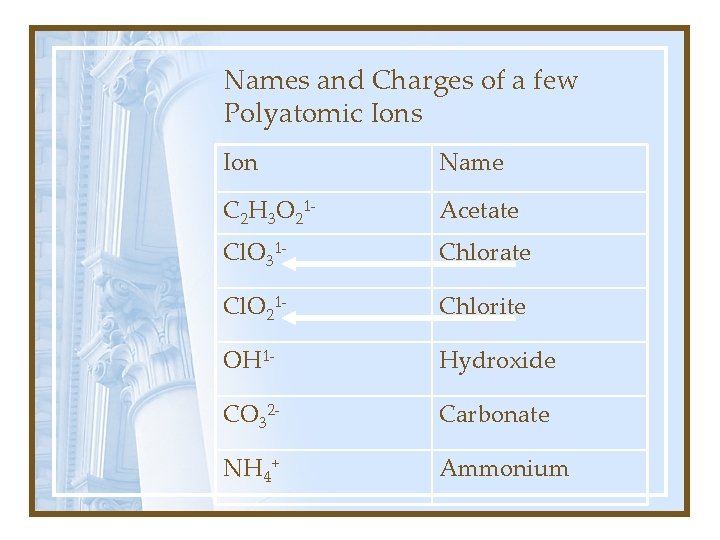
Names and Charges of a few Polyatomic Ions Ion Name C 2 H 3 O 21 - Acetate Cl. O 31 - Chlorate Cl. O 21 - Chlorite OH 1 - Hydroxide CO 32 - Carbonate NH 4+ Ammonium

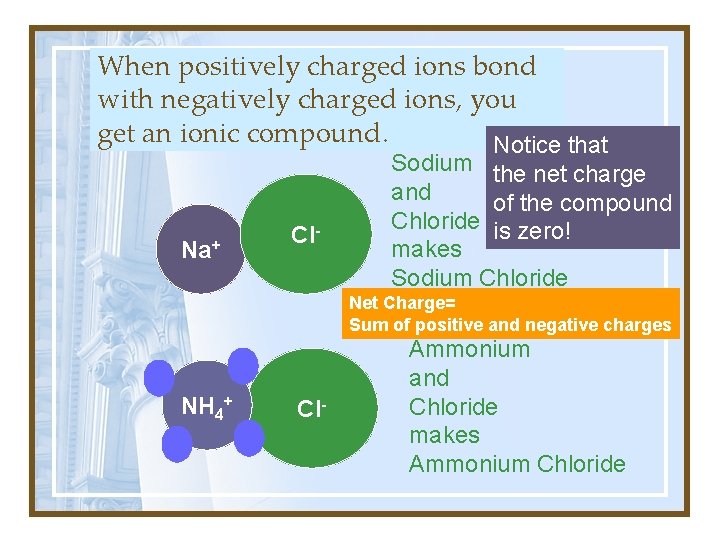
When positively charged ions bond with negatively charged ions, you get an ionic compound. Notice that Na+ Cl- Sodium the net charge and of the compound Chloride is zero! makes Sodium Chloride Net Charge= Sum of positive and negative charges NH 4+ Cl- Ammonium and Chloride makes Ammonium Chloride

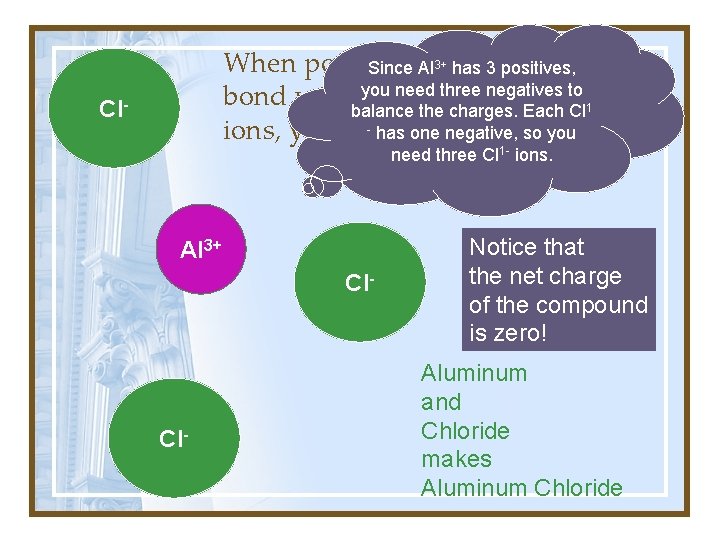
3+ has 3 positives, When positively ions Since Alcharged you need three negatives to bond withbalance negatively charged the charges. Each Cl 1 - has negative, so you ions, you get anone ionic compound. Cl- need three Cl 1 - ions. Al 3+ Cl- Notice that the net charge of the compound is zero! Aluminum and Chloride makes Aluminum Chloride

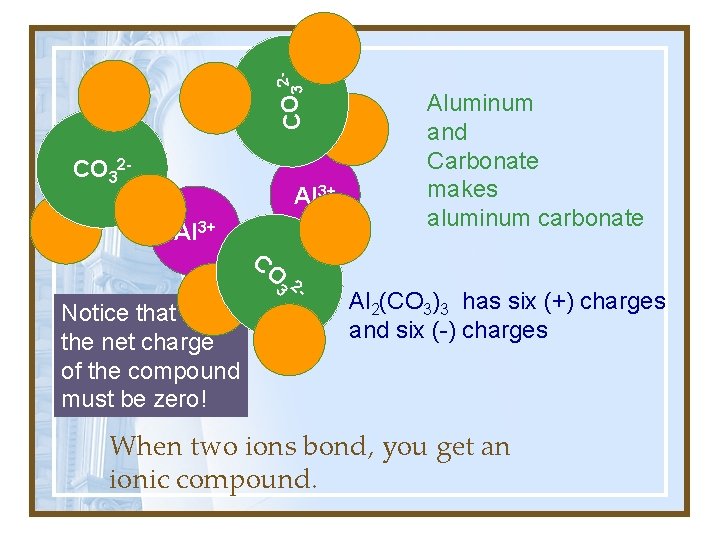
2 O C 3 CO 32 - Al 3+ CO 3 2 - Notice that the net charge of the compound must be zero! Aluminum and Carbonate makes aluminum carbonate Al 2(CO 3)3 has six (+) charges and six (-) charges When two ions bond, you get an ionic compound.

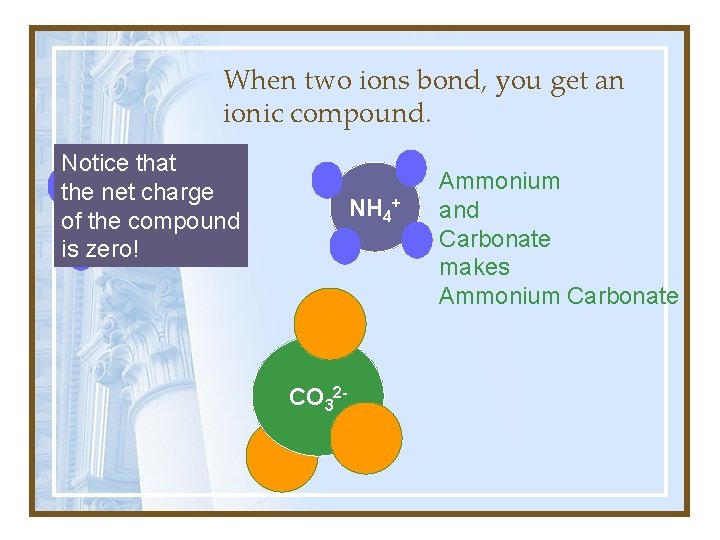
When two ions bond, you get an ionic compound. Notice that the net charge + NH 4 of the compound is zero! NH 4+ CO 32 - Ammonium and Carbonate makes Ammonium Carbonate

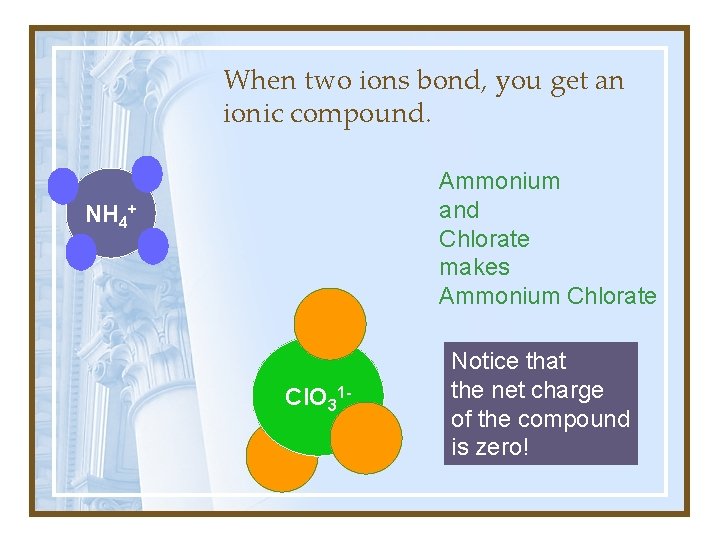
When two ions bond, you get an ionic compound. Ammonium and Chlorate makes Ammonium Chlorate NH 4+ Cl. O 31 - Notice that the net charge of the compound is zero!

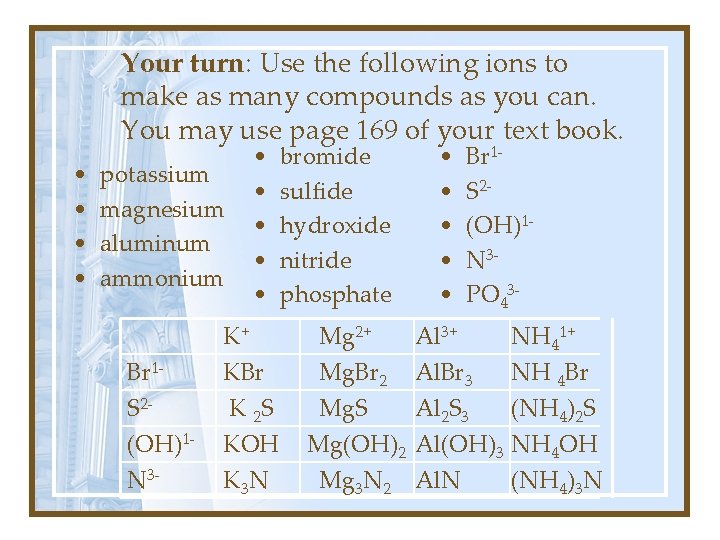
Your turn: Use the following ions to make as many compounds as you can. You may use page 169 of your text book. • • potassium magnesium aluminum ammonium Br 1 S 2(OH)1 N 3 - • • • K+ KBr K 2 S KOH K 3 N bromide sulfide hydroxide nitride phosphate Mg 2+ Mg. Br 2 Mg. S Mg(OH)2 Mg 3 N 2 • • • Br 1 S 2(OH)1 N 3 PO 43 - Al 3+ Al. Br 3 Al 2 S 3 Al(OH)3 Al. N NH 41+ NH 4 Br (NH 4)2 S NH 4 OH (NH 4)3 N