Chemical Formulas Subscripts represent relative numbers of elements
Chemical Formulas • Subscripts represent relative numbers of elements present • (Parentheses) separate complexes or substituted elements – Fe(OH)3 – Fe bonded to 3 separate OH groups – (Mg, Fe)Si. O 4 – Olivine group – mineral composed of 0 -100 % of Mg, 100 -Mg% Fe
Stoichiometry • Some minerals contain varying amounts of 2+ elements which substitute for each other • Solid solution – elements substitute in the mineral structure on a sliding scale, defined in terms of the end members – species which contain 100% of one of the elements
Chemical heterogeneity • Matrix containing ions a mineral forms in contains many different ions/elements – sometimes they get into the mineral • Ease with which they do this: – Solid solution: ions which substitute easily form a series of minerals with varying compositions (olivine series how easily Mg (forsterite) and Fe (fayalite) swap…) – Impurity defect: ions of lower quantity or that have a harder time swapping get into the structure
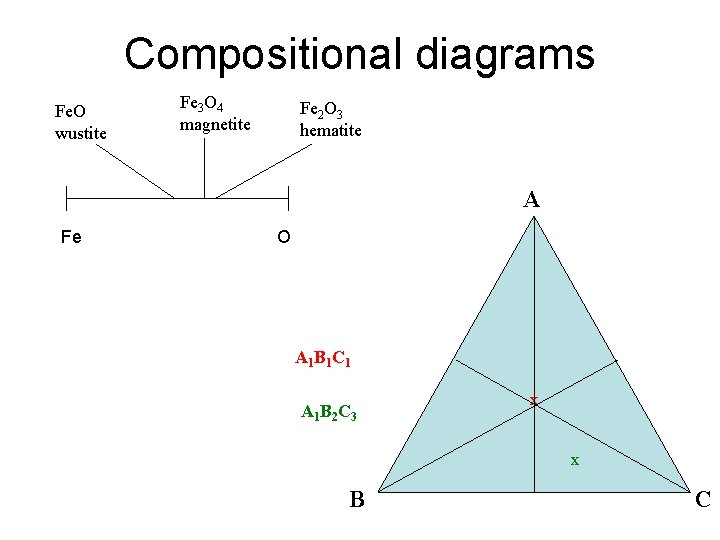
Compositional diagrams Fe. O wustite Fe 3 O 4 magnetite Fe 2 O 3 hematite A Fe O A 1 B 1 C 1 A 1 B 2 C 3 x x B C
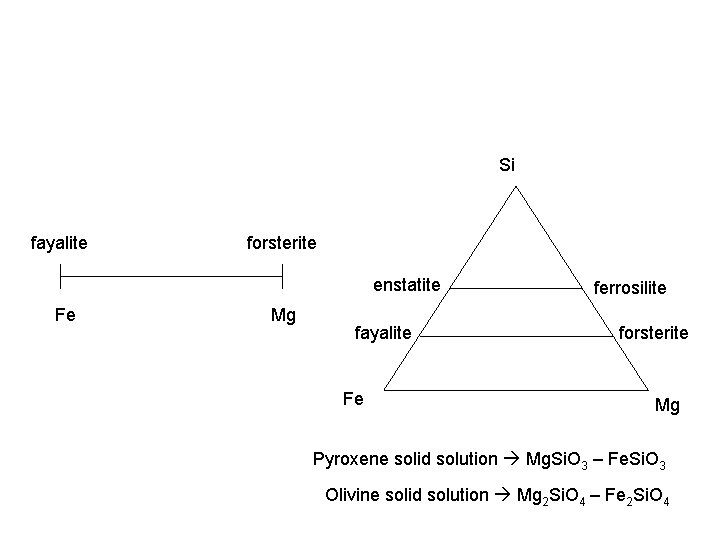
Si fayalite forsterite enstatite Fe Mg fayalite Fe ferrosilite forsterite Mg Pyroxene solid solution Mg. Si. O 3 – Fe. Si. O 3 Olivine solid solution Mg 2 Si. O 4 – Fe 2 Si. O 4
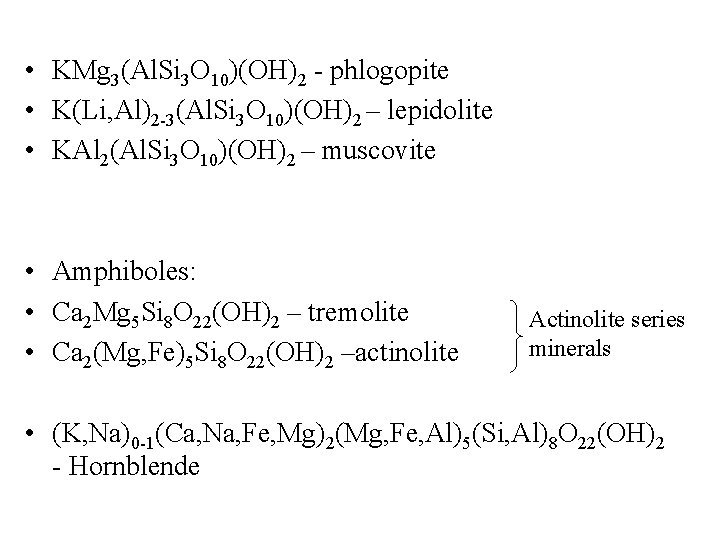
• KMg 3(Al. Si 3 O 10)(OH)2 - phlogopite • K(Li, Al)2 -3(Al. Si 3 O 10)(OH)2 – lepidolite • KAl 2(Al. Si 3 O 10)(OH)2 – muscovite • Amphiboles: • Ca 2 Mg 5 Si 8 O 22(OH)2 – tremolite • Ca 2(Mg, Fe)5 Si 8 O 22(OH)2 –actinolite Actinolite series minerals • (K, Na)0 -1(Ca, Na, Fe, Mg)2(Mg, Fe, Al)5(Si, Al)8 O 22(OH)2 - Hornblende
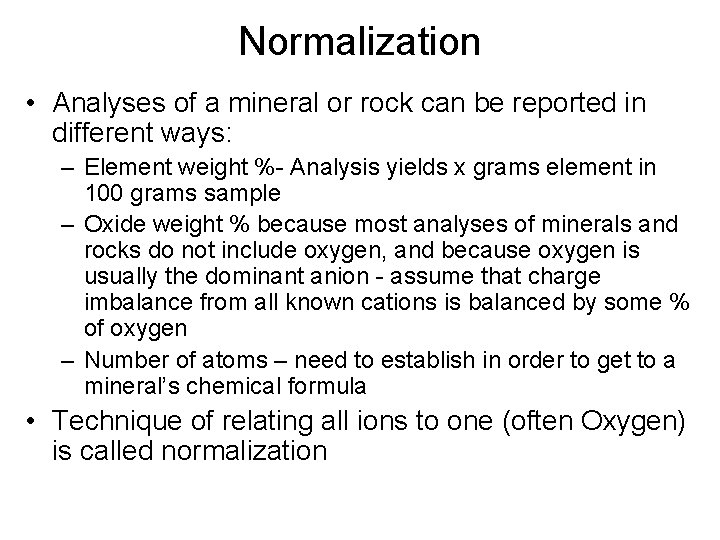
Normalization • Analyses of a mineral or rock can be reported in different ways: – Element weight %- Analysis yields x grams element in 100 grams sample – Oxide weight % because most analyses of minerals and rocks do not include oxygen, and because oxygen is usually the dominant anion - assume that charge imbalance from all known cations is balanced by some % of oxygen – Number of atoms – need to establish in order to get to a mineral’s chemical formula • Technique of relating all ions to one (often Oxygen) is called normalization
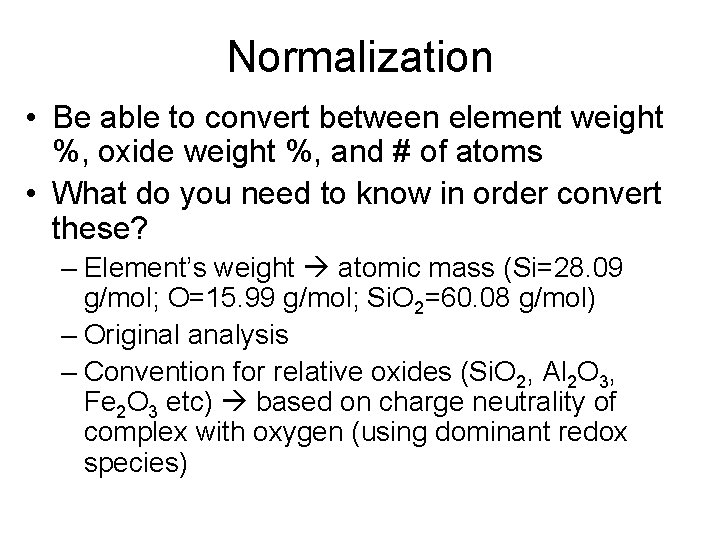
Normalization • Be able to convert between element weight %, oxide weight %, and # of atoms • What do you need to know in order convert these? – Element’s weight atomic mass (Si=28. 09 g/mol; O=15. 99 g/mol; Si. O 2=60. 08 g/mol) – Original analysis – Convention for relative oxides (Si. O 2, Al 2 O 3, Fe 2 O 3 etc) based on charge neutrality of complex with oxygen (using dominant redox species)
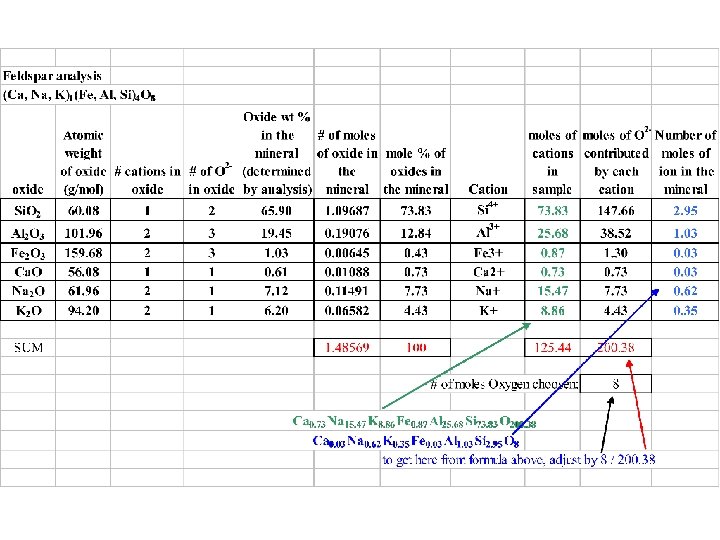
Normalization example • Start with data from quantitative analysis: weight percent of oxide in the mineral • Convert this to moles of oxide per 100 g of sample by dividing oxide weight percent by the oxide’s molecular weight • ‘Fudge factor’ from Perkins Box 1. 5, pg 22: is process called normalization – where we divide the number of moles of one thing by the total moles all species/oxides then are presented relative to one another
- Slides: 10