Chemical Formulas represent compounds Chemical formulas are composed









- Slides: 30


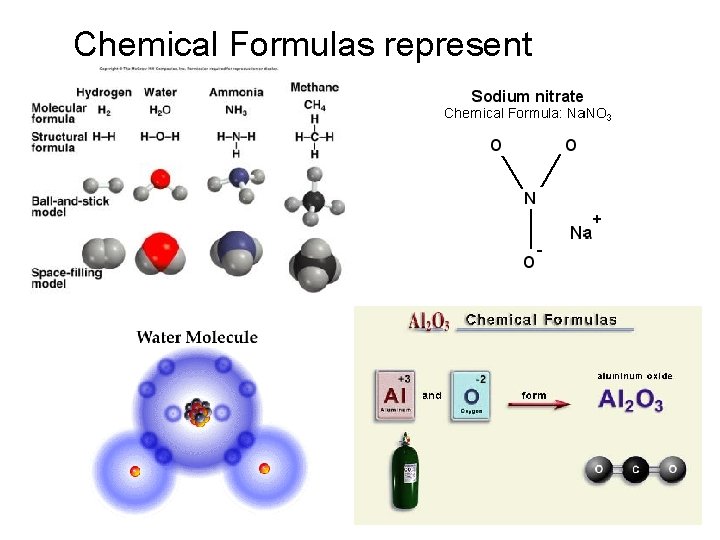
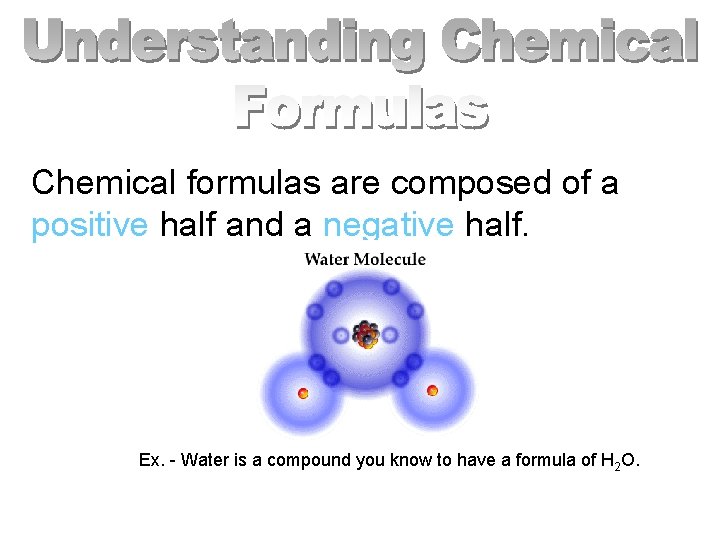
Chemical Formulas represent compounds.

Chemical formulas are composed of a positive half and a negative half. Ex. - Water is a compound you know to have a formula of H 2 O.

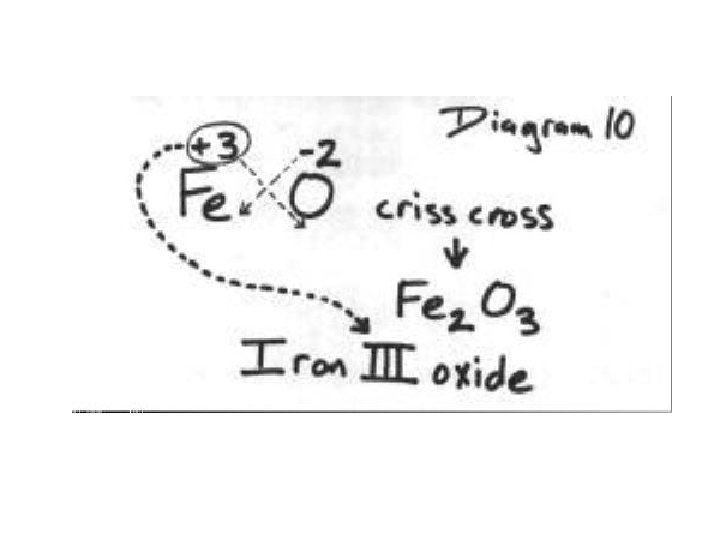
The easiest way to think of writing chemical formulas is to use the oxidation number (without the + or -) of one element as the subscript of the other element. (look at Periodic Table to get oxidation numbers) +2 -1

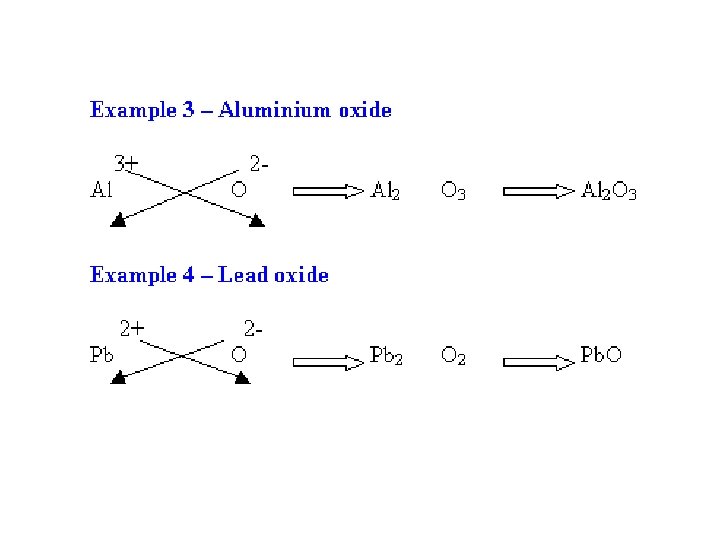
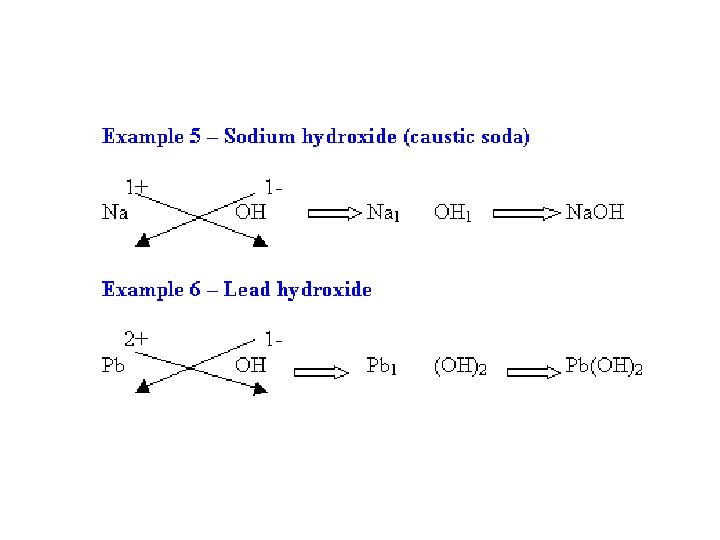
Cross over the oxidation numbers without the charges!!! +2 -1

2 REMINDER: DO NOT write a subscript of 1. Reduce the subscripts if needed.





NAMING COMPOUNDS • Your ability to name compounds and write formula’s hinges on your ability to recognize whether a compound is Ionic or Molecular.

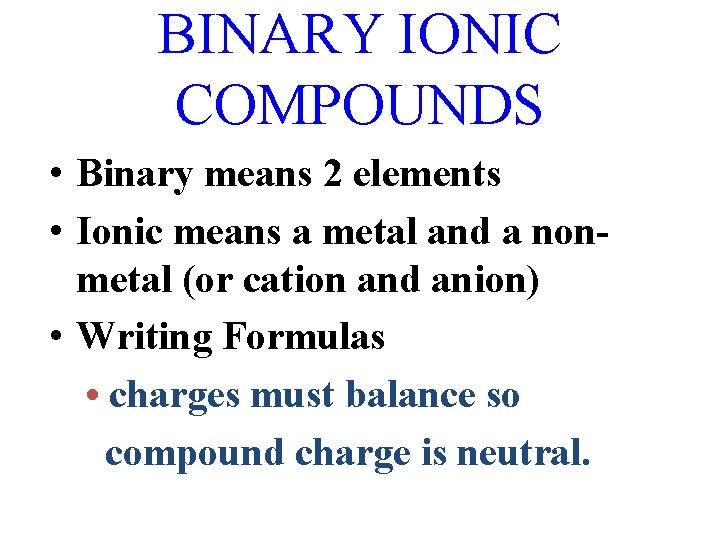
BINARY IONIC COMPOUNDS • Binary means 2 elements • Ionic means a metal and a nonmetal (or cation and anion) • Writing Formulas • charges must balance so compound charge is neutral.

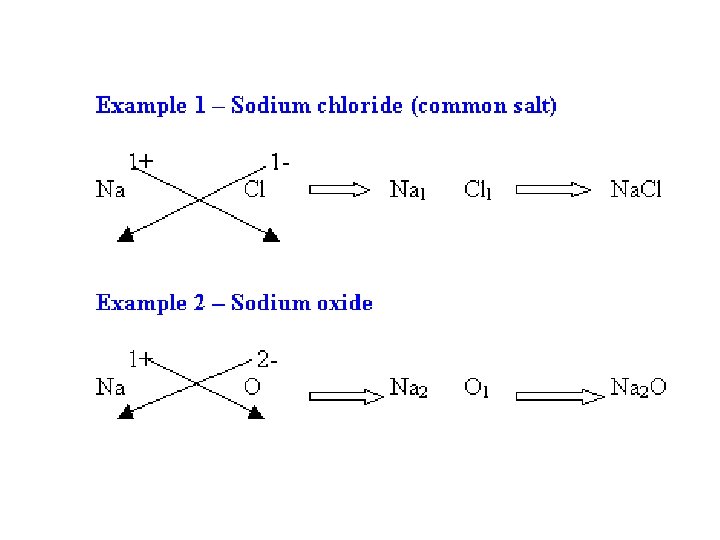
BINARY IONIC COMPOUNDS • Writing Formulas from Names • 1 st word = CATION • 2 nd word = ANION name with ide ending.

BINARY IONIC COMPOUNDS • Na. Br • Mg. F 2 • Sodium Bromide • Magnesium Fluoride

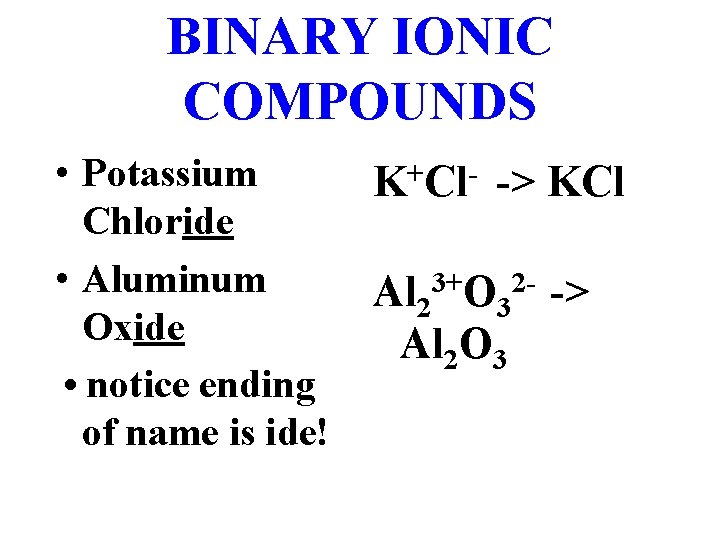
BINARY IONIC COMPOUNDS • Potassium Chloride • Aluminum Oxide • notice ending of name is ide! + K Cl -> KCl Al 23+O 32 - -> Al 2 O 3

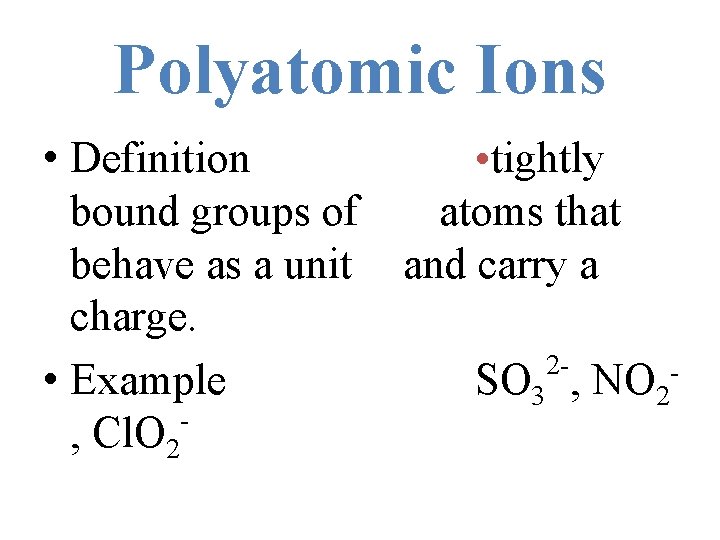
Polyatomic Ions • Definition bound groups of behave as a unit charge. • Example , Cl. O 2 • tightly atoms that and carry a 2 SO 3 , NO 2 -

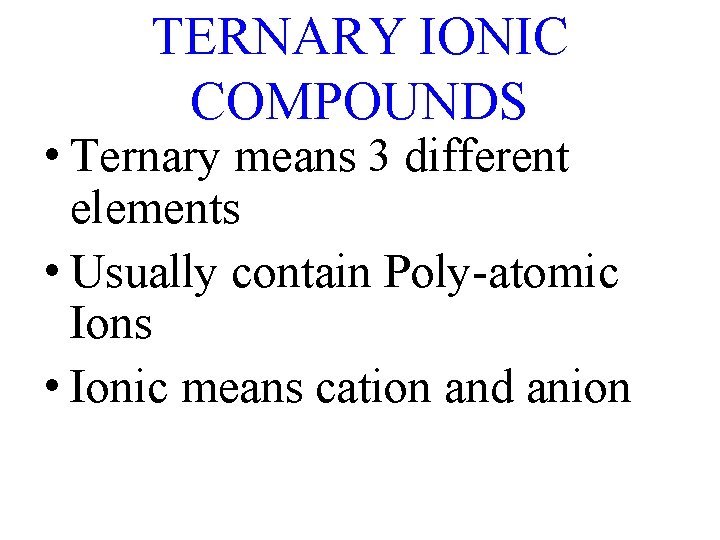
TERNARY IONIC COMPOUNDS • Ternary means 3 different elements • Usually contain Poly-atomic Ions • Ionic means cation and anion

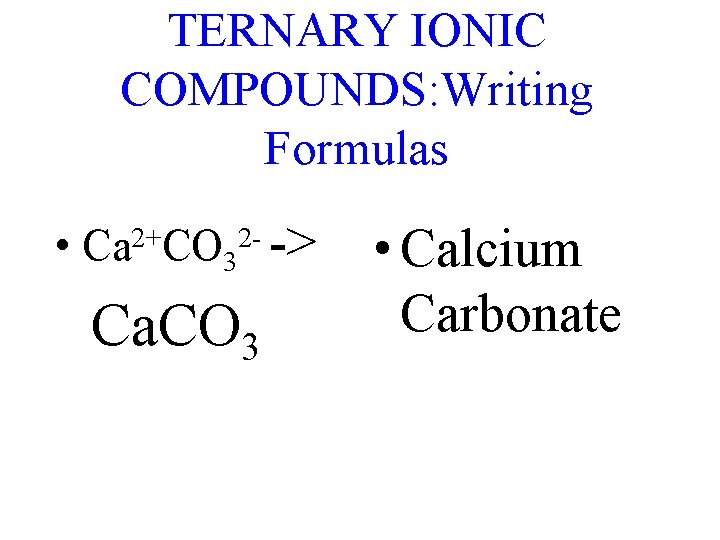
TERNARY IONIC COMPOUNDS: Writing Formulas • Ca 2+CO 32 - -> Ca. CO 3 • Calcium Carbonate

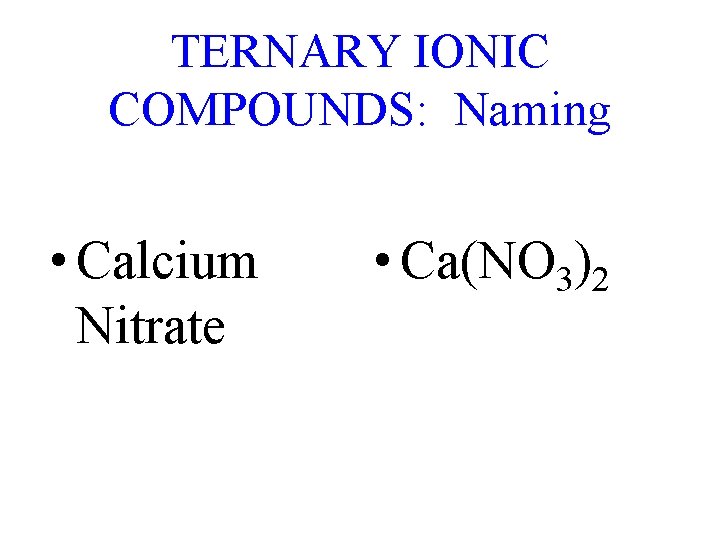
TERNARY IONIC COMPOUNDS: Naming • Calcium Nitrate • Ca(NO 3)2

Naming with Transition Metals • First word = CATION • Second word = ANION • You need to determine what charge is on the transition metal if more than one exists.

Naming Transition Metals • Copper (I) Oxide • Cu 2 O

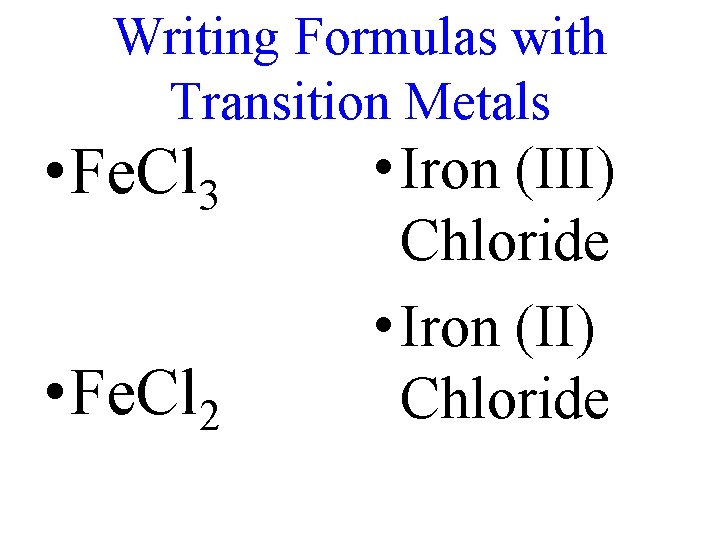
Writing Formulas with Transition Metals • Fe. Cl 3 • Fe. Cl 2 • Iron (III) Chloride • Iron (II) Chloride

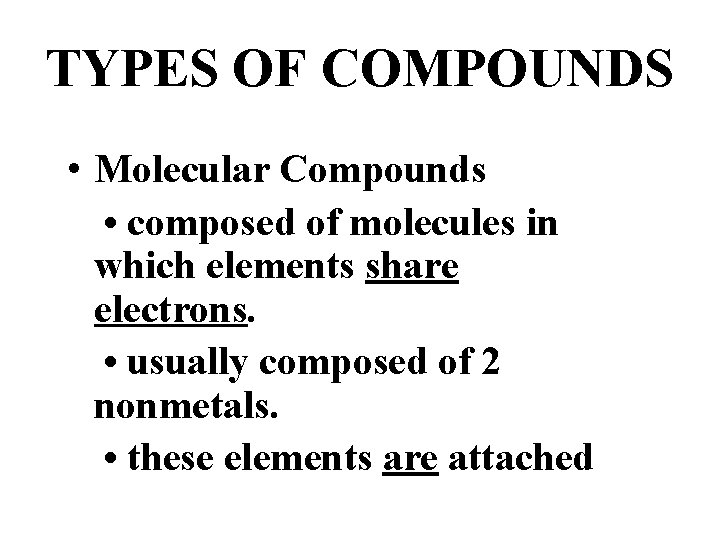
TYPES OF COMPOUNDS • Molecular Compounds • composed of molecules in which elements share electrons. • usually composed of 2 nonmetals. • these elements are attached

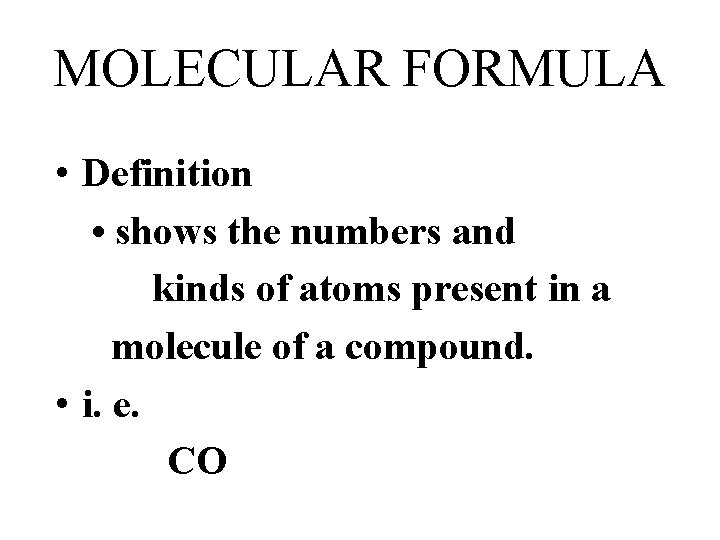
MOLECULAR FORMULA • Definition • shows the numbers and kinds of atoms present in a molecule of a compound. • i. e. CO

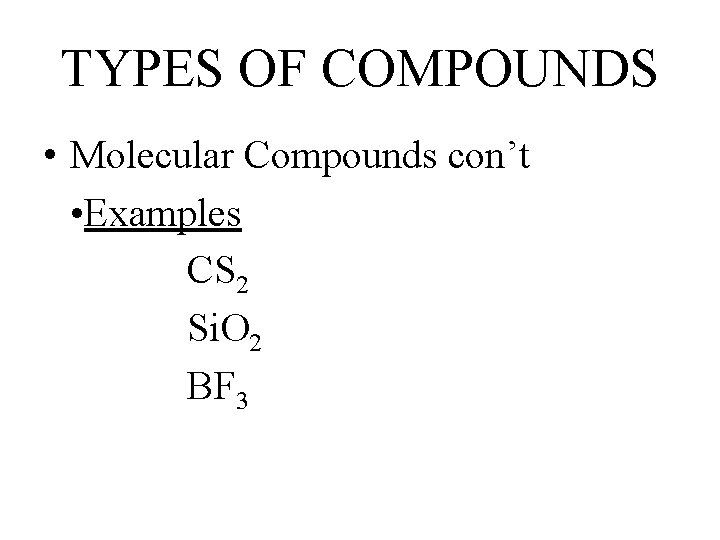
TYPES OF COMPOUNDS • Molecular Compounds con’t • Examples CS 2 Si. O 2 BF 3

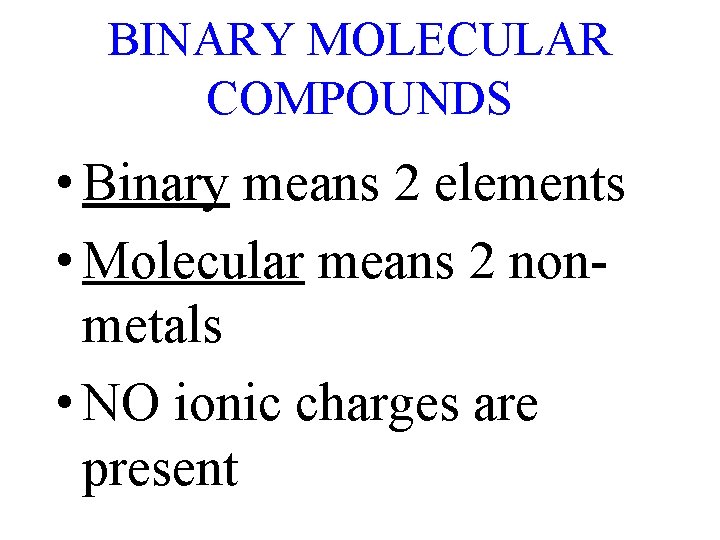
BINARY MOLECULAR COMPOUNDS • Binary means 2 elements • Molecular means 2 nonmetals • NO ionic charges are present

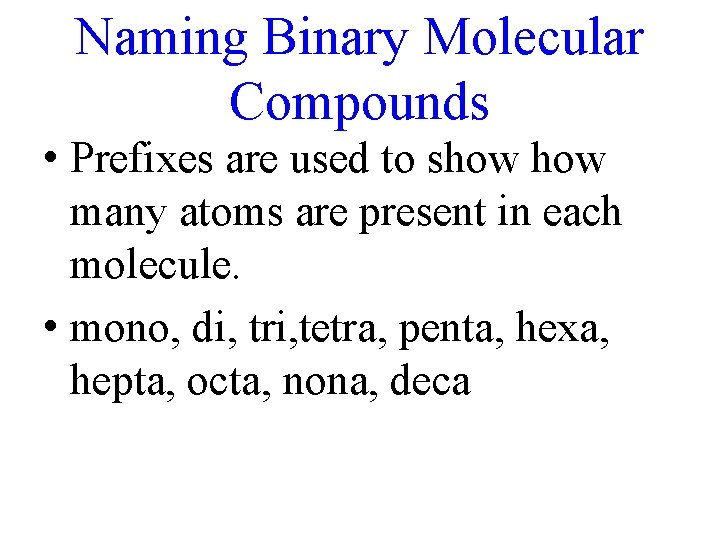
Naming Binary Molecular Compounds • Prefixes are used to show many atoms are present in each molecule. • mono, di, tri, tetra, penta, hexa, hepta, octa, nona, deca

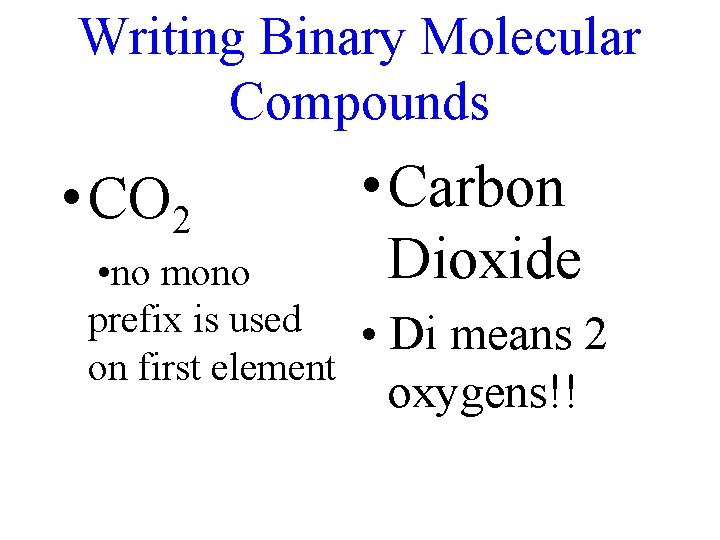
Writing Binary Molecular Compounds • CO 2 • no mono prefix is used on first element • Carbon Dioxide • Di means 2 oxygens!!

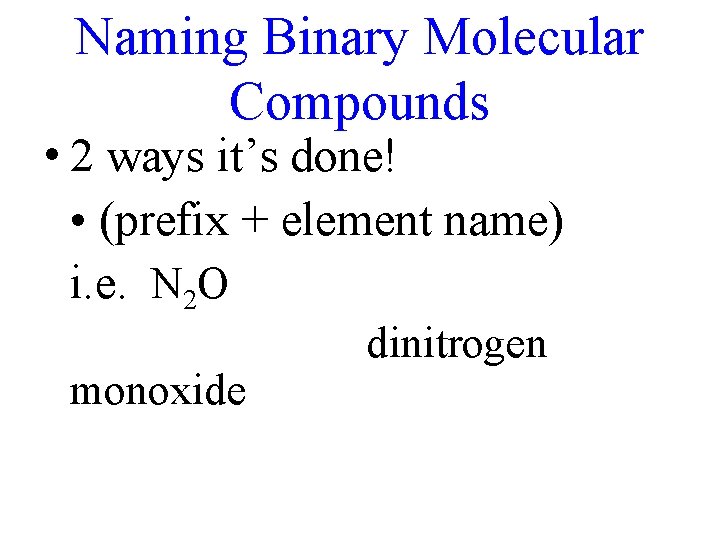
Naming Binary Molecular Compounds • 2 ways it’s done! • (prefix + element name) i. e. N 2 O monoxide dinitrogen

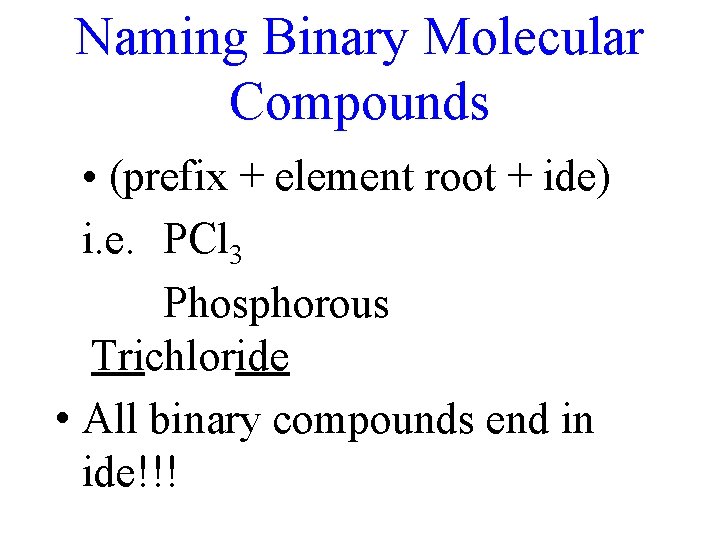
Naming Binary Molecular Compounds • (prefix + element root + ide) i. e. PCl 3 Phosphorous Trichloride • All binary compounds end in ide!!!