Chemical Formulas for Ionic Compounds Mixed Review Putting







- Slides: 9

Chemical Formulas for Ionic Compounds Mixed Review Putting It All Together!

Chemical Bonds Ø Three things happen to electrons in a chemical bond: Gain, lose, share Ø Ionic Bond (This Unit) = gain and loss Ø Covalent Bond (Next Unit) = share Ø How do we name compounds with ionic bonds and write their formulas? Ø Must correctly name the cation and anion, then follow the rules.

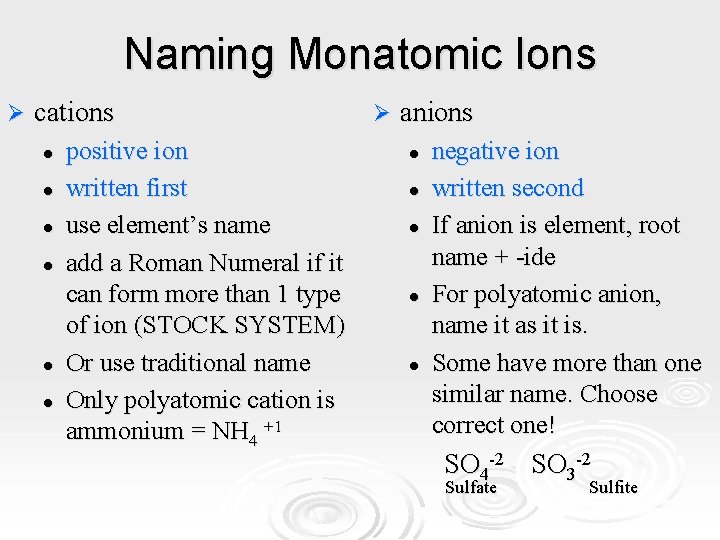
Naming Monatomic Ions Ø cations l l l positive ion written first use element’s name add a Roman Numeral if it can form more than 1 type of ion (STOCK SYSTEM) Or use traditional name Only polyatomic cation is ammonium = NH 4 +1 Ø anions l l l negative ion written second If anion is element, root name + -ide For polyatomic anion, name it as it is. Some have more than one similar name. Choose correct one! SO 4 -2 SO 3 -2 Sulfate Sulfite

Cross-Over Rule Ø Crossing Over l l write two ions with correct charges make the anion’s charge, the cation’s subscript make the cation’s charge, the anion’s subscript simplify the ratio if needed

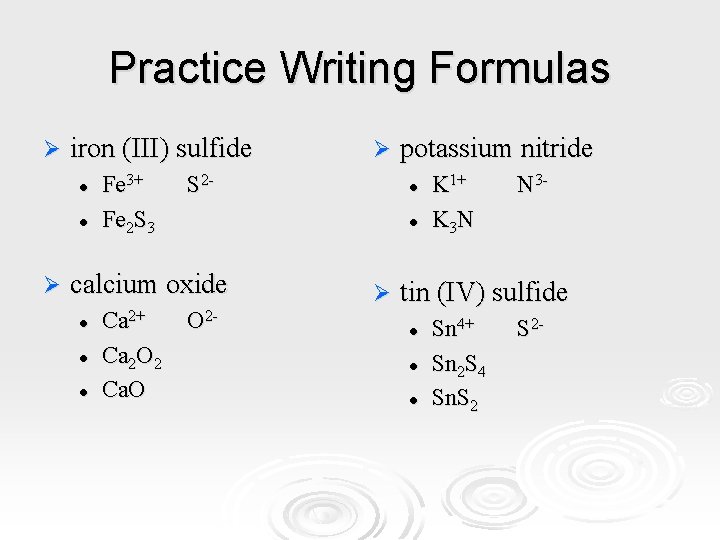
Practice Writing Formulas Ø iron (III) sulfide l l Ø Fe 3+ Fe 2 S 3 S 2 - l l Ca 2+ Ca 2 O 2 Ca. O potassium nitride l l calcium oxide l Ø O 2 - Ø K 1+ K 3 N N 3 - tin (IV) sulfide l l l Sn 4+ Sn 2 S 4 Sn. S 2 -

Practice Writing Formulas Ø sodium permanganate l l Ø Na 1+ Mn. O 41 Na. Mn. O 4 iron (II) chlorite l l Ø Fe 2+ Cl. O 21 Fe(Cl. O 2)2 ammonium acetate l l Ø NH 41+ CH 3 COO 1 NH 4 CH 3 COO calcium carbonate l l Ca 2+ CO 32 Ca. CO 3

Practice Naming Ø KNO 3 l l potassium nitrate from K 1+ and NO 31 - Ø Cu(OH)2 l l l copper (II) hydroxide cuprous hydroxide from Cu 2+ and OH 1 - Ø NH 4 Br l l ammonium bromide from NH 41+ and Br 1 - Ø Ca. SO 4 l l calcium sulfate from Ca 2+ and SO 42 -

Practice Naming Ø Al 2 (SO 2)3 l aluminum sulfite Ø Ni. F 2 l l Ø Mg. I 2 l magnesium iodide Nickel (II) fluoride Nickelous fluoride

Memorize for the Test Valences for Groups 1, 2, 13, 15, 16, and 17 Ø Zinc is +2 Silver is +1 Ø Be able to identify which traditional name goes with each element that has more than one valence. Most are obvious! Ø The ones that are NOT obvious: Ø Aurous/Auric = Gold (Au) Cuprous/Cupric = Copper (Cu) Ferric/Ferrous = Iron (Fe) Plumbous/Plumbic = Lead (Pb) Stannous/Stannic = Tin (Sn)