Chemical Formulas and Equations Letters form words In







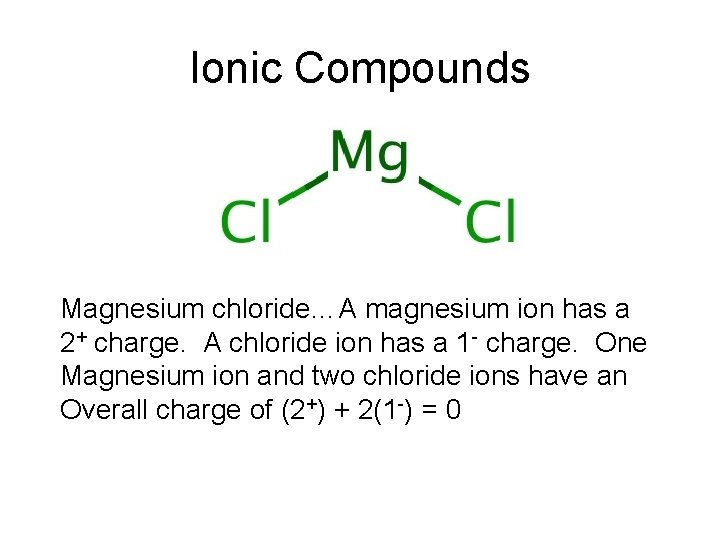















- Slides: 46

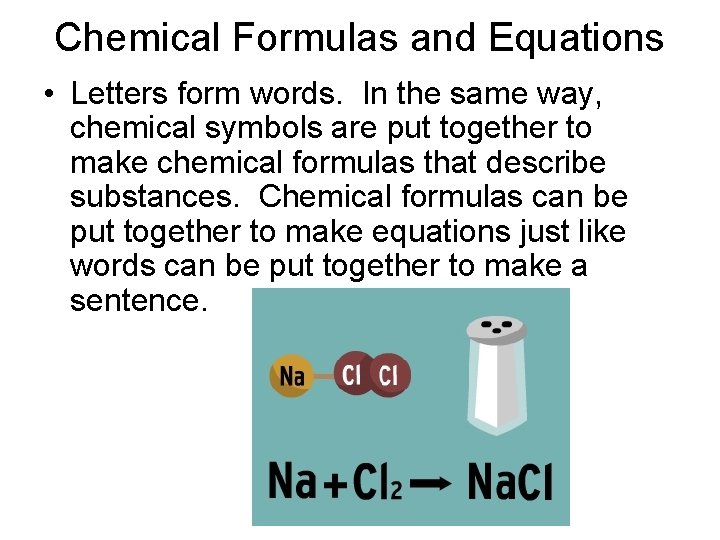
Chemical Formulas and Equations • Letters form words. In the same way, chemical symbols are put together to make chemical formulas that describe substances. Chemical formulas can be put together to make equations just like words can be put together to make a sentence.

Question 1 • What is a chemical formula?

Chemical Formula • Shorthand way to use chemical symbols and numbers to represent a substance. • A chemical formula shows how many atoms of each kind are present in a molecule. • Na. Cl • H 2 O • CO 2

Question 2 • What is a subscript?

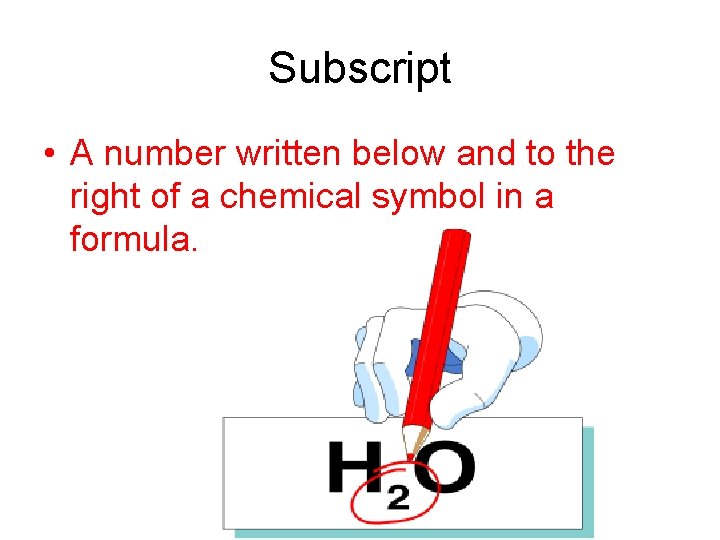
Subscript • A number written below and to the right of a chemical symbol in a formula.

Subscript • If there is no subscript, only one atom of the element is present. Na. Cl has one atom of sodium and one atom of chlorine.

Question 3 • What is a coefficient?

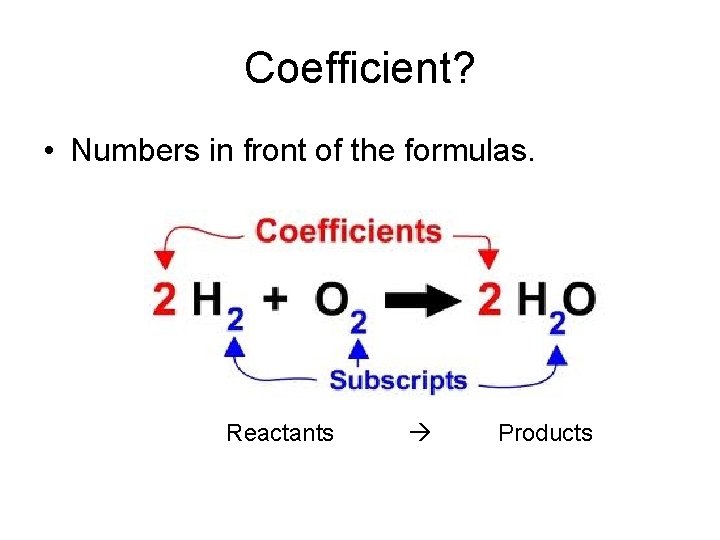
Coefficient? • Numbers in front of the formulas. Reactants Products

Question 4 • How do you write the chemical formula for a covalent compound?

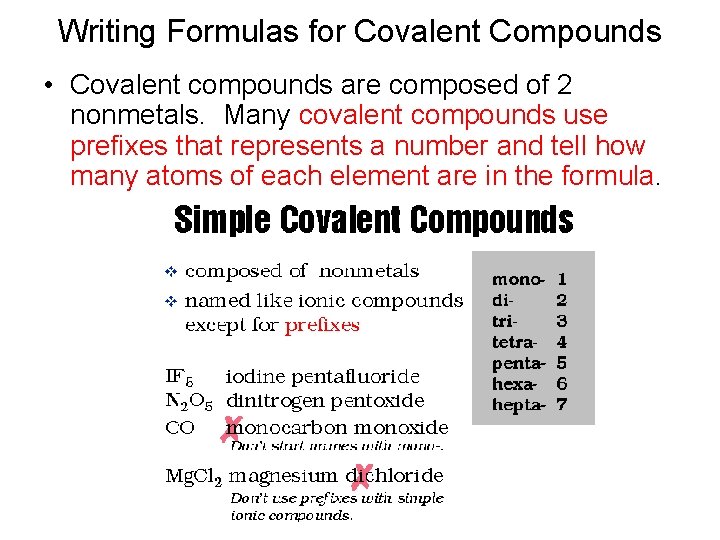
Writing Formulas for Covalent Compounds • Covalent compounds are composed of 2 nonmetals. Many covalent compounds use prefixes that represents a number and tell how many atoms of each element are in the formula.

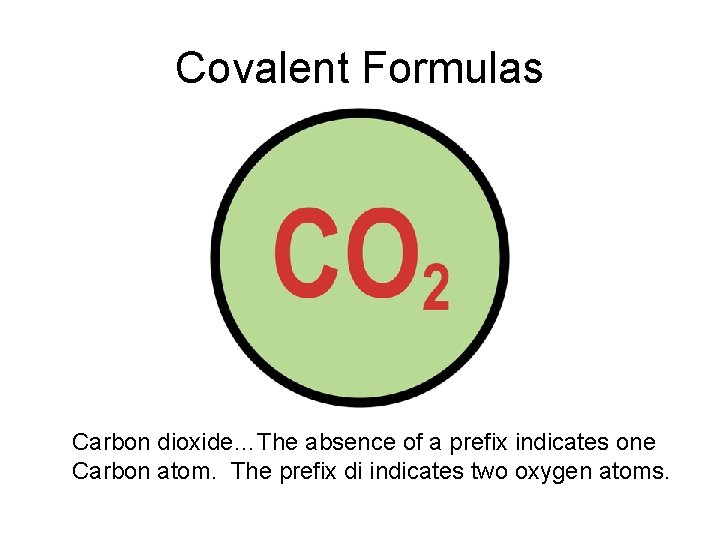
Covalent Formulas Carbon dioxide…The absence of a prefix indicates one Carbon atom. The prefix di indicates two oxygen atoms.

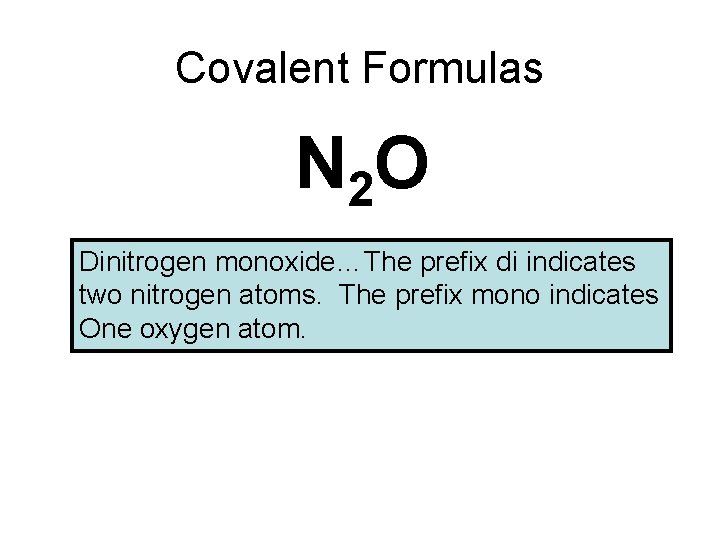
Covalent Formulas N 2 O Dinitrogen monoxide…The prefix di indicates two nitrogen atoms. The prefix mono indicates One oxygen atom.

Question 5 • How do you write ionic compounds?

Ionic Compounds Sodium Chloride…A sodium ion has a 1+ charge. A chloride ion has a 1 - charge. One sodium ion and one chloride ion have an overall charge of 0.
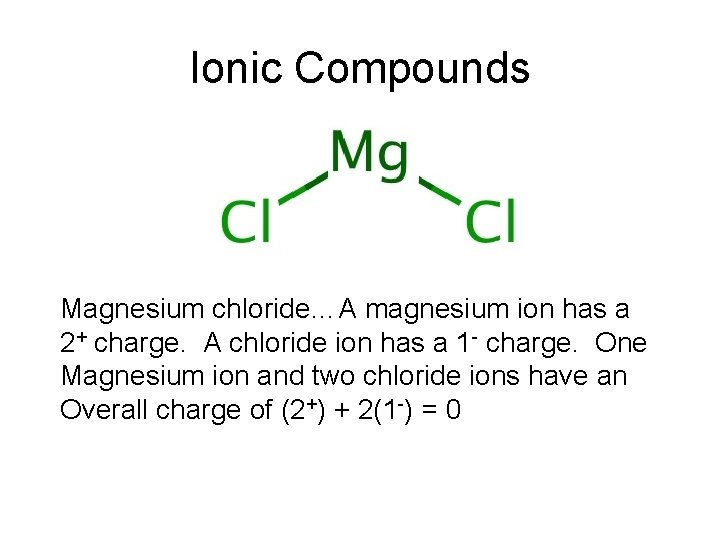
Ionic Compounds Magnesium chloride…A magnesium ion has a 2+ charge. A chloride ion has a 1 - charge. One Magnesium ion and two chloride ions have an Overall charge of (2+) + 2(1 -) = 0

Question 6 • What is a chemical equation?

Chemical Equation • A shorthand description of a chemical reaction using chemical formulas and symbols. C + O 2 CO 2

Question 7 • What are reactants?

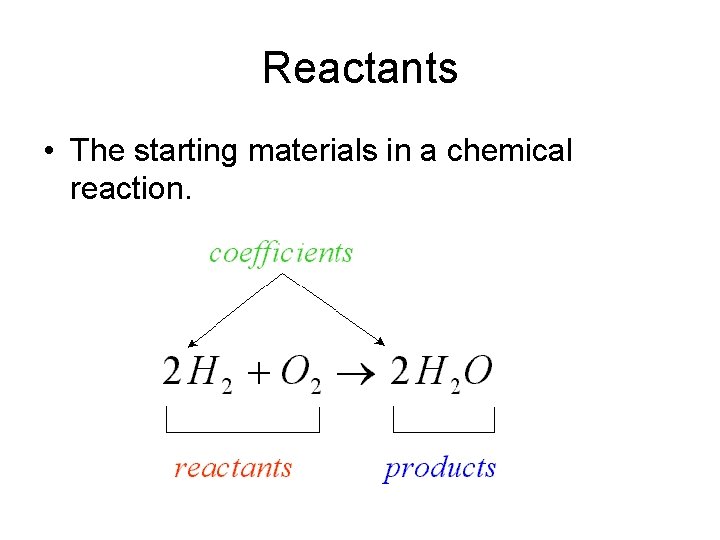
Reactants • The starting materials in a chemical reaction.

Question 8 • What are the products?

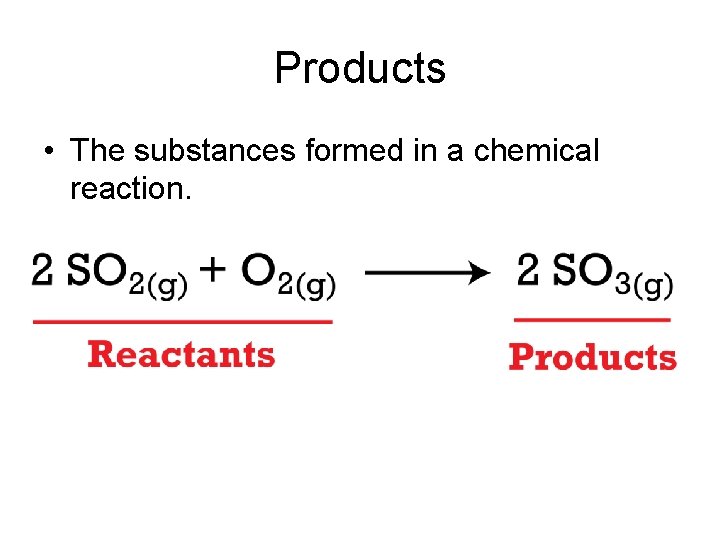
Products • The substances formed in a chemical reaction.

Question 9 • Where do the reactants appear in a chemical reaction?

Chemical Reactions • Reactants appear before the arrow and products appear after the arrow.

Question 10 • What separates the formulas of the reactants from the formulas of the products?

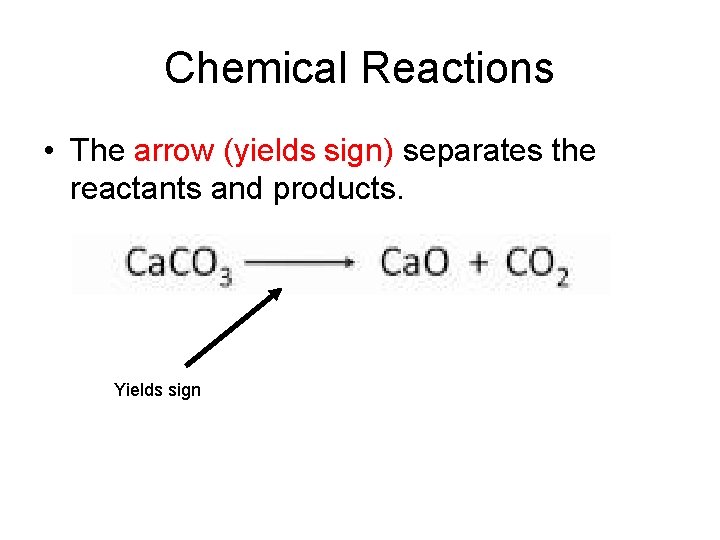
Chemical Reactions • The arrow (yields sign) separates the reactants and products. Yields sign

Question 11 • What separates the formulas?

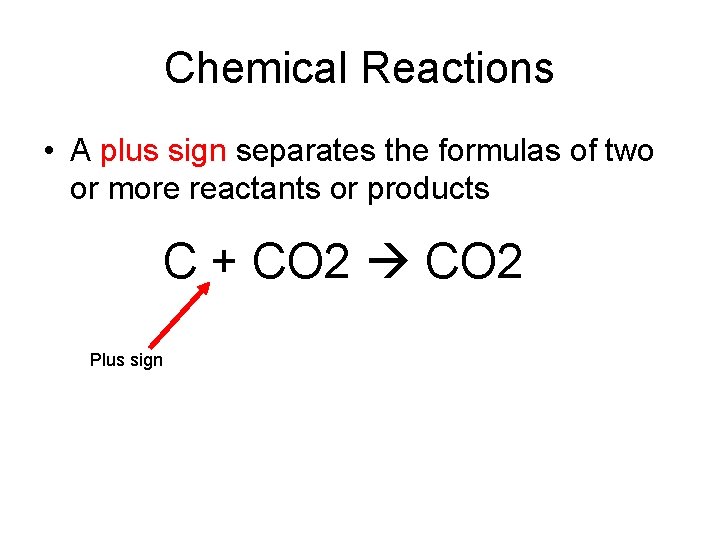
Chemical Reactions • A plus sign separates the formulas of two or more reactants or products C + CO 2 Plus sign

Video • Go to folder. . . click “chemical equations…law of conservation of mass. ”

The Importance of Accuracy • CO 2 is a colorless, odorless gas you exhale. • CO is a colorless, odorless, and poisonous gas. • Co is an element. CO 2 CO Co

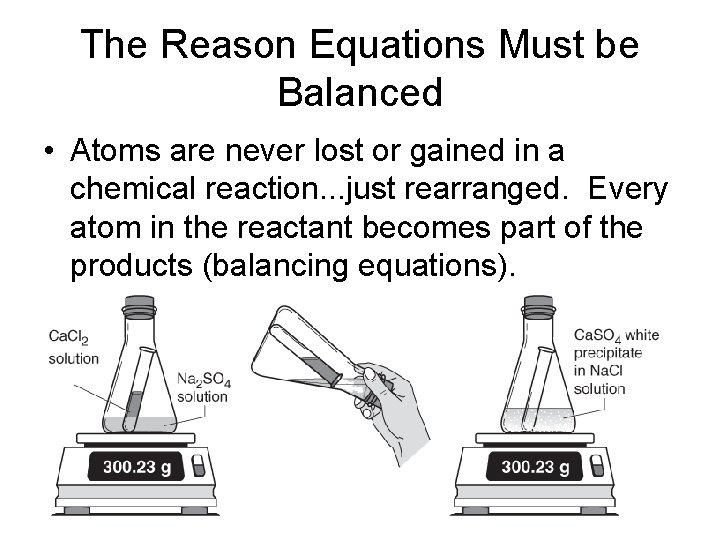
The Reason Equations Must be Balanced • Atoms are never lost or gained in a chemical reaction. . . just rearranged. Every atom in the reactant becomes part of the products (balancing equations).

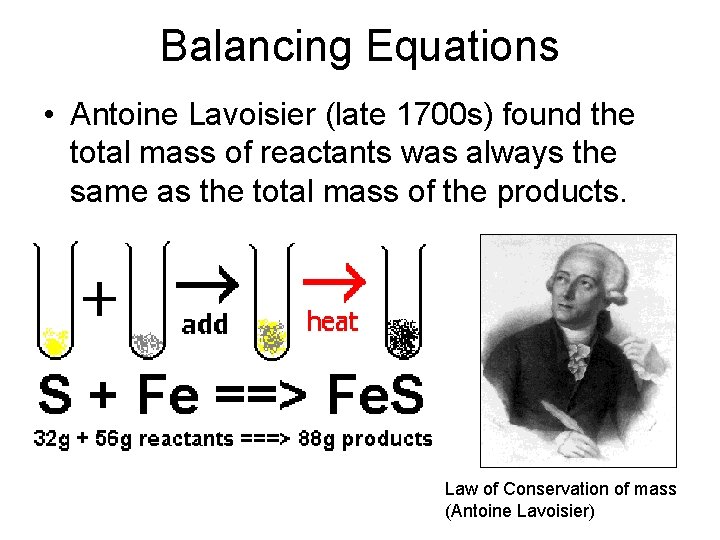
Balancing Equations • Antoine Lavoisier (late 1700 s) found the total mass of reactants was always the same as the total mass of the products. Law of Conservation of mass (Antoine Lavoisier)

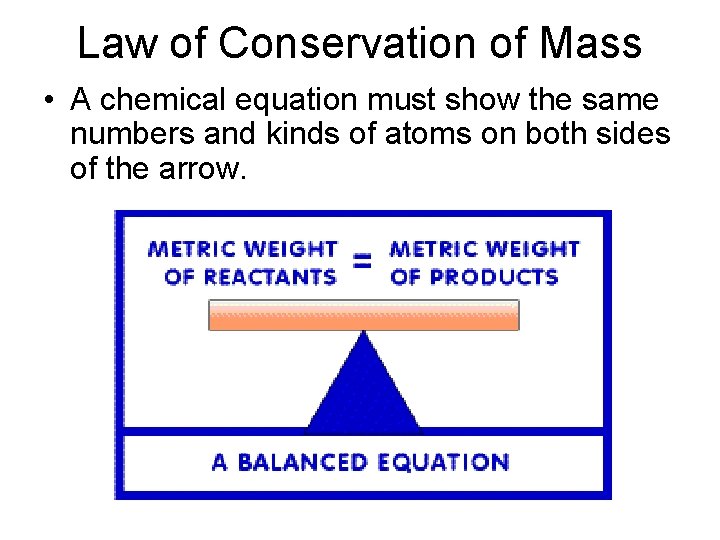
Law of Conservation of Mass • Mass is neither created nor destroyed in ordinary chemical and physical changes.

Law of Conservation of Mass • A chemical equation must show the same numbers and kinds of atoms on both sides of the arrow.

Question 12 • What is a coefficient?

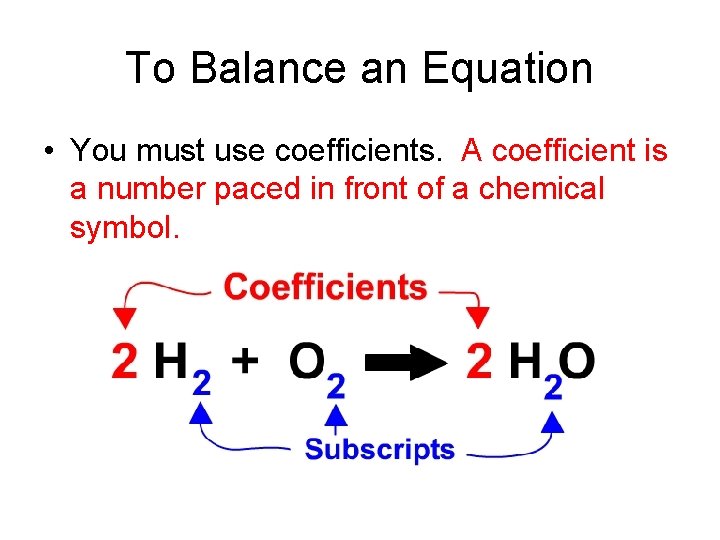
To Balance an Equation • You must use coefficients. A coefficient is a number paced in front of a chemical symbol.

Video • Go to folder and click #2 “Balancing Equations forming water”

To Balance an Equation • How do you balance an equation?

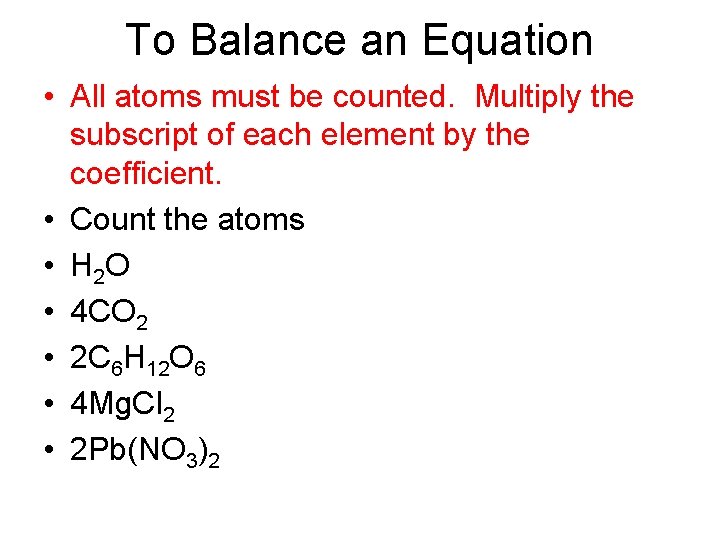
To Balance an Equation • All atoms must be counted. Multiply the subscript of each element by the coefficient. • Count the atoms • H 2 O • 4 CO 2 • 2 C 6 H 12 O 6 • 4 Mg. Cl 2 • 2 Pb(NO 3)2

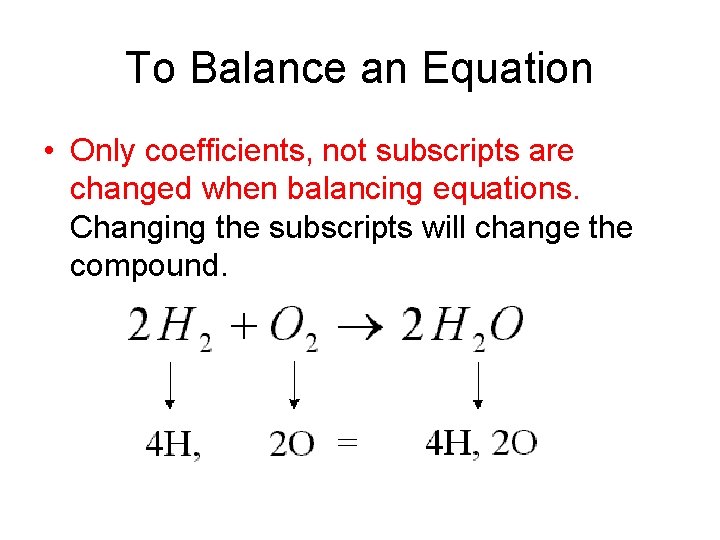
To Balance an Equation • Only coefficients, not subscripts are changed when balancing equations. Changing the subscripts will change the compound.

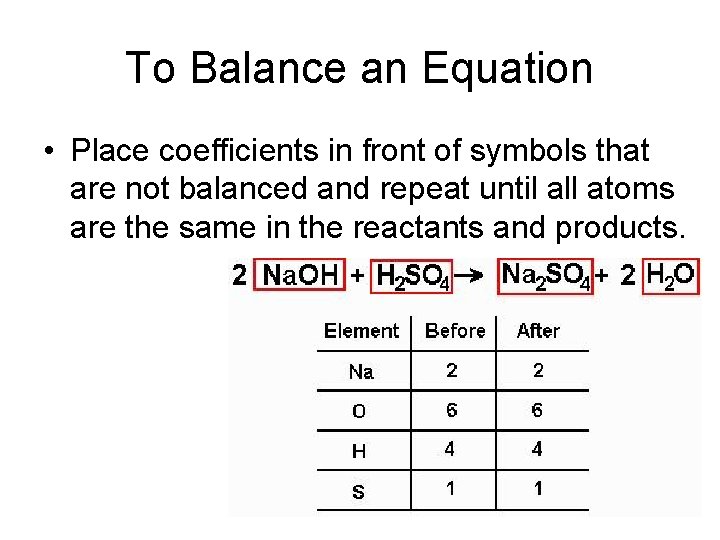
To Balance an Equation • Place coefficients in front of symbols that are not balanced and repeat until all atoms are the same in the reactants and products.

Video • Go to folder and click #3 “Balancing Equations Forming Rust. ”

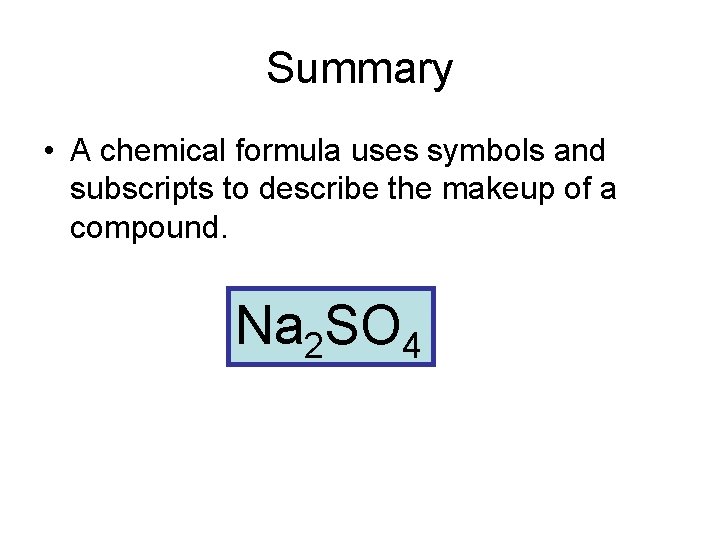
Summary • A chemical formula uses symbols and subscripts to describe the makeup of a compound. Na 2 SO 4

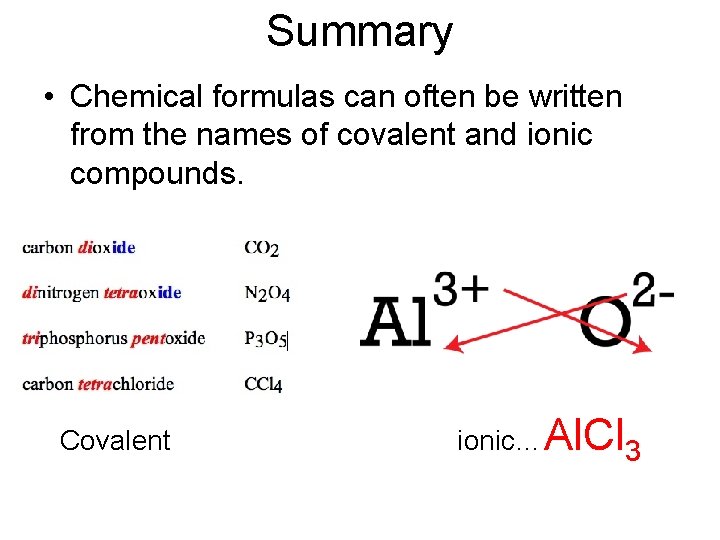
Summary • Chemical formulas can often be written from the names of covalent and ionic compounds. Covalent ionic… Al. Cl 3

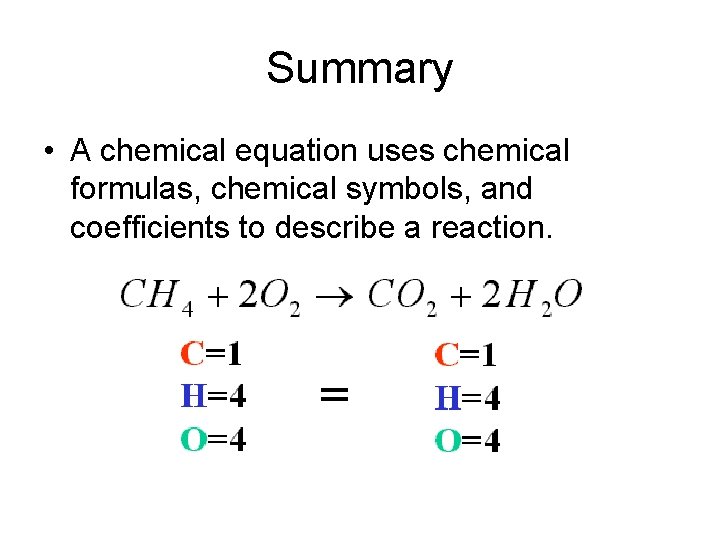
Summary • A chemical equation uses chemical formulas, chemical symbols, and coefficients to describe a reaction.

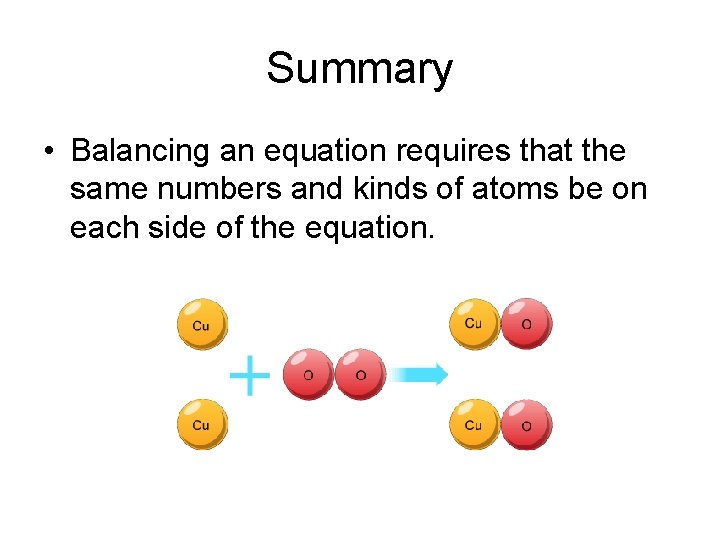
Summary • Balancing an equation requires that the same numbers and kinds of atoms be on each side of the equation.

Summary • A balanced equation illustrates the law of conservation of mass: mass is neither created nor destroyed during ordinary physical and chemical changes.