Chemical Families The Noble Gases Group VIIIA Also


Chemical Families

The Noble Gases Group VIIIA Also called Inert Gases Have a full outer shell of electrons ◦ Called a stable octet Very un-reactive

The Halogens Group VIIA 7 Valence electrons ◦ One electron short of stability

The Alkali Metals Group IA 1 Valence electron ◦ One electron over stability Very reactive Largest atoms most reactive

The Alkaline Earth Metals Group IIA 2 Valence electrons ◦ Two electrons over stability Reactive, but not as much as Alkali’s

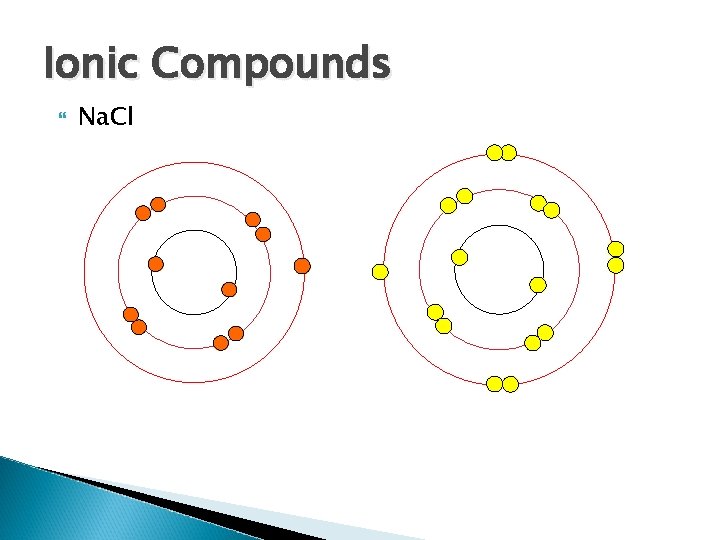
Ions Atoms who have gained or lost an electron Atoms gain or lose electrons to have a stable octet! ◦ Cl- Needs one electron to complete octet. When it gains an electron it becomes negatively charged. ◦ Na+ Is looking to give away an electron. When it gives up an electron it becomes positively charged.
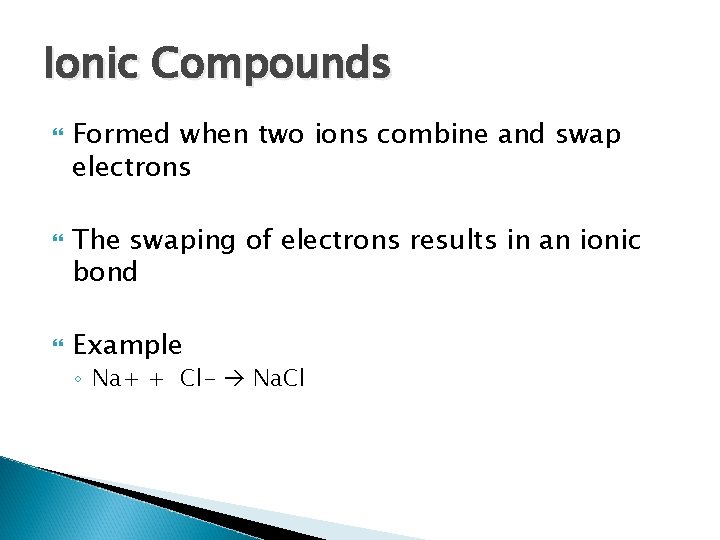
Ionic Compounds Formed when two ions combine and swap electrons The swaping of electrons results in an ionic bond Example ◦ Na+ + Cl- Na. Cl

Remember…. . Atoms want to get to a stable octet Some atoms (mostly metals) have to give up e- to get a stable octet (ex. Na). They have more p+ than e- so they become positively charged.

Some atoms (Mostly non-metals) want to take e- to get a stable octet (ex. Cl) they have more e- than p+ so they become negatively charged. (count p+ vs. e-) Since opposites attract the positive ion attracts and bonds to the negative ion and so an ionic bond is formed.
Ionic Compounds Na. Cl
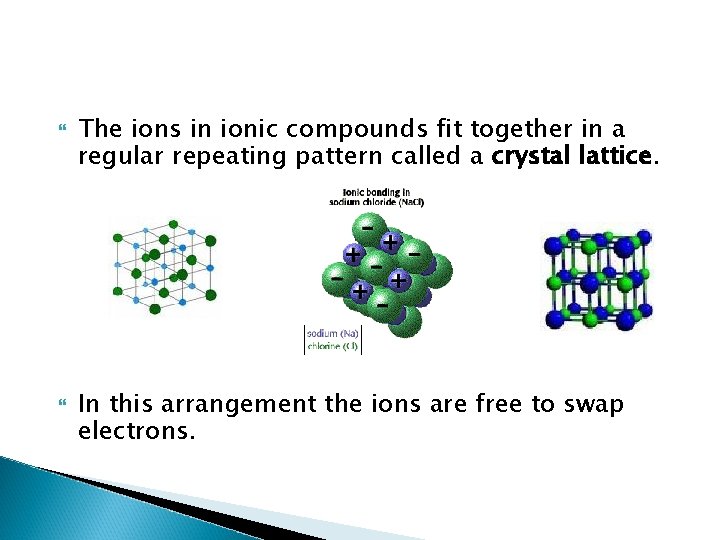
The ions in ionic compounds fit together in a regular repeating pattern called a crystal lattice. In this arrangement the ions are free to swap electrons.

Charges Usually when elements want to form ionic bonds their charges come into play. It is important to make the ionic bond have a neutral charge. (match up the correct number of negatives with positives) The charge of an element is the number of electrons they are willing to give up or the number they need. ( see Periodic Table)

Naming Ionic and Covalent Compounds

Ionic Compounds Naming: ◦ Must include a metal and a non-metal ion. ◦ 1 st ion is named according to the PT ◦ 2 nd ion – the ending is changed to –ide. Na. Cl Mg. F 2

Na. F Mg. Cl 2 potassium fluoride magnesium iodide
Ionic Compounds Writing Formulas: ◦ Write the symbols of each ion present ◦ Obtain their charges ◦ Write the formula to obtain a neutral charge using the “criss-cross method” Lithium bromide: Copper chloride:
Covalent Compounds are when atoms share electrons. This is where both elements use the same electron as part of their octet. It only happens between non-metals and non -metals
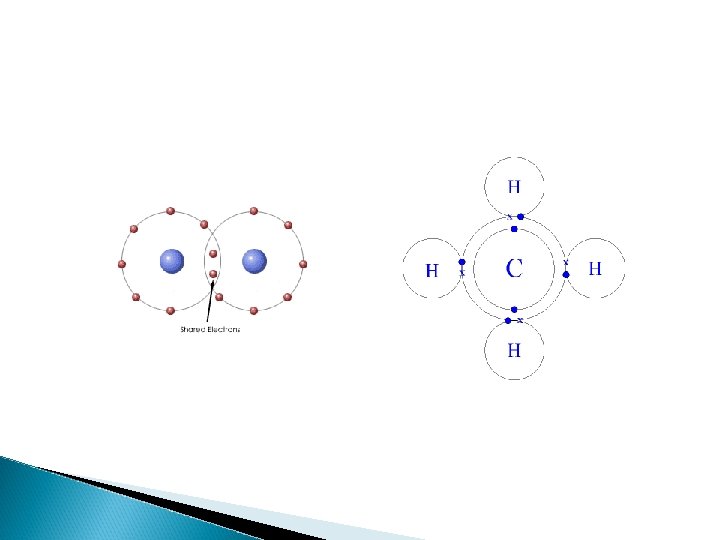
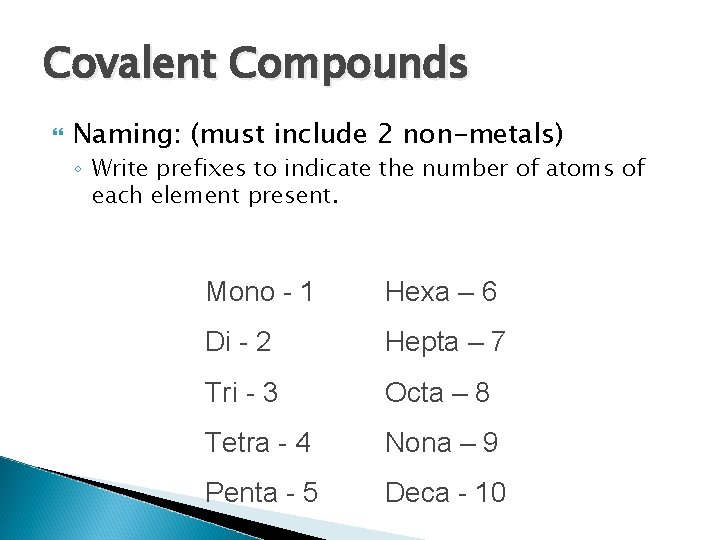
Covalent Compounds Naming: (must include 2 non-metals) ◦ Write prefixes to indicate the number of atoms of each element present. Mono - 1 Hexa – 6 Di - 2 Hepta – 7 Tri - 3 Octa – 8 Tetra - 4 Nona – 9 Penta - 5 Deca - 10
Covalent Compounds Examples ◦ CO 2 ◦ NO 3 ◦ P 4 O 10

Covalent Compounds Writing formulas: ◦ nitrogen monoxide ◦ carbon tetrachloride

Ionic Bond - A ionic bond is a chemical bond that it made by losing or gaining electrons and having the atoms. This causes the opposite ions to attract and stay together. Only a metal and a nonmetal can bond this way. Covalent Bond - Covalent bonds use a chemical bonding and can only be done by two non-metals. Covalent bonds share electrons and when each of the atoms share 1 electron, 2 electrons, 3 electrons, it is called Single bonding, Double bonding, and triple bonding, respectively. Also, atoms that bond to themselves do so with covalent bonds. This creates a strong bond between them.
- Slides: 26