Chemical Equilibrium The study of reactions that occur
Chemical Equilibrium The study of reactions that occur in both directions.

So far with reactions…. n Looked at reactions that go to completion n Used stoichiometry for calculation of many quantities n Looked at spontaneity and rates of reactions

Now… n Reactions can be reversible – They reach a state of equilibrium
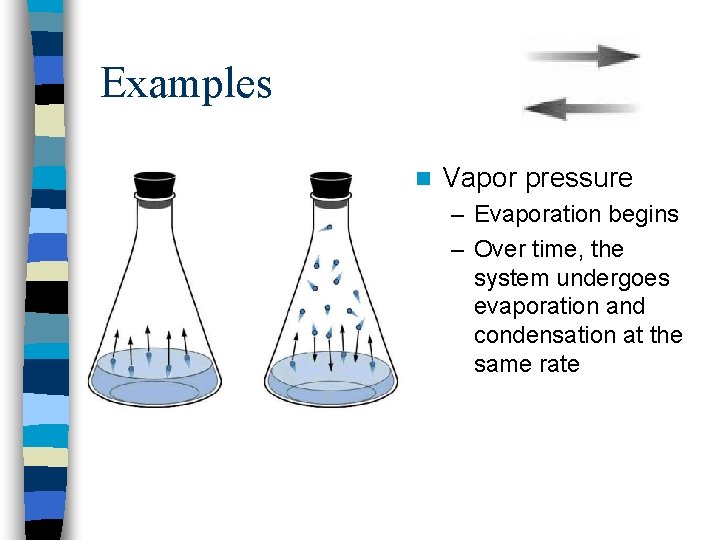
Examples n Vapor pressure – Evaporation begins – Over time, the system undergoes evaporation and condensation at the same rate
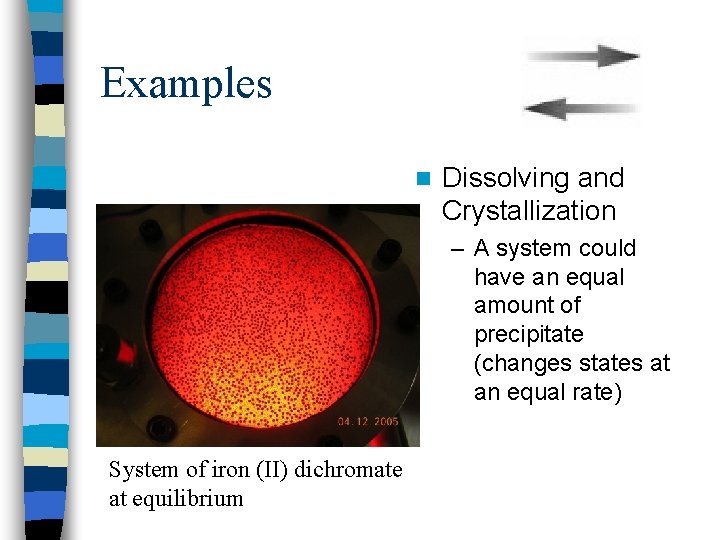
Examples n Dissolving and Crystallization – A system could have an equal amount of precipitate (changes states at an equal rate) System of iron (II) dichromate at equilibrium

Examples NO 2 Equilibrium!! N 2 O 4 n NO 2 (g) + NO 2 (g) N 2 O 4 (g) – NO 2 = dark brown, N 2 O 4 = colorless – Ultimately ends up somewhere in between Equlibrium Change in Action
Equilibrium Defined n Concentration of products and reactants remain constant over time – The reaction is reversible (can go both directions) – The rate of forward reaction equals the rate of the reverse reaction – Dynamic!! (looks the same when taking a snapshot, but constantly moving back and forth)
![Demo, then Graph of Equilibrium [R] [P] Time Demo, then Graph of Equilibrium [R] [P] Time](http://slidetodoc.com/presentation_image_h2/03d483bae6611cc52fdeea70ea1d467b/image-8.jpg)
Demo, then Graph of Equilibrium [R] [P] Time
![Explaining the Graph [R] What’s happening to the products? n What’s happening to the Explaining the Graph [R] What’s happening to the products? n What’s happening to the](http://slidetodoc.com/presentation_image_h2/03d483bae6611cc52fdeea70ea1d467b/image-9.jpg)
Explaining the Graph [R] What’s happening to the products? n What’s happening to the reactants? n Which one is favored? n Different reactions have different equilibria… n [P] Time
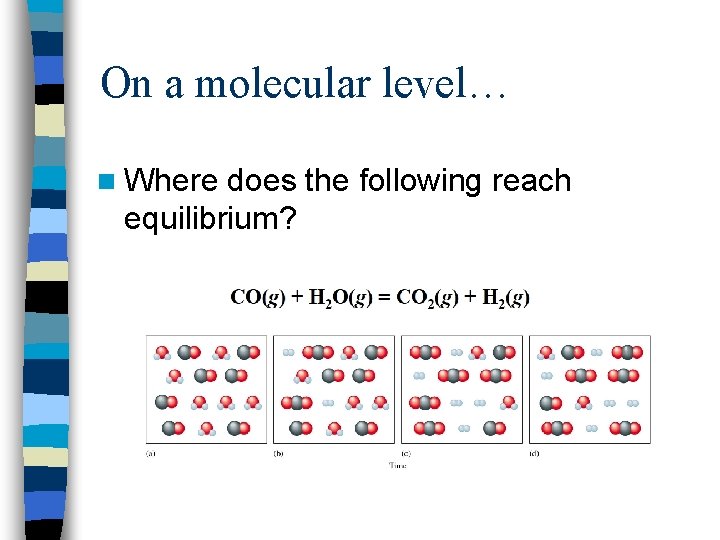
On a molecular level… n Where does the following reach equilibrium?

Equilibrium Expressions n Idea by Guldberg and Waage (1864) – Called the Law of Mass Action – Given a reaction: a. A + b. B c. C + d. D
![Equilibrium Expressions n a. A + b. B c. C + d. D [C]c Equilibrium Expressions n a. A + b. B c. C + d. D [C]c](http://slidetodoc.com/presentation_image_h2/03d483bae6611cc52fdeea70ea1d467b/image-12.jpg)
Equilibrium Expressions n a. A + b. B c. C + d. D [C]c [D]d = [A]a [B]b Equilibrium constant MUST use concentrations of products over reactants coefficients of balanced equation become exponents
![Equilibrium Expressions [C]c [D]d = [A]a [B]b n Works only for GASES and IONS Equilibrium Expressions [C]c [D]d = [A]a [B]b n Works only for GASES and IONS](http://slidetodoc.com/presentation_image_h2/03d483bae6611cc52fdeea70ea1d467b/image-13.jpg)
Equilibrium Expressions [C]c [D]d = [A]a [B]b n Works only for GASES and IONS – No pure solids or liquids included n Example – C 3 H 8 (g) + O 2 (g) CO 2 (g) + H 2 O (g) [CO 2]3 [H 2 O]4 = [C 3 H 8] [O 2]5
Equilibrium Expressions n What if it has solids or liquids? – Called heterogeneous equilibrium – The concentration of solids and liquids is assumed to always remain constant, so they are not included… – Example: Ca (s) + O 2 (g) Ca. O (s)
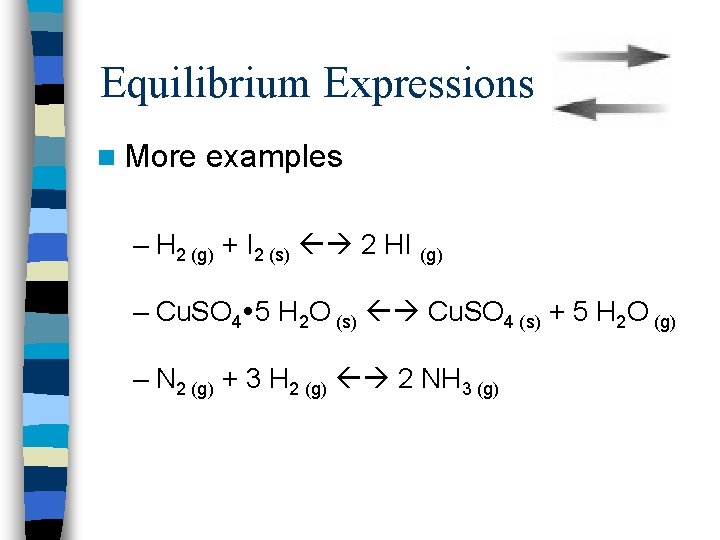
Equilibrium Expressions n More examples – H 2 (g) + I 2 (s) 2 HI (g) – Cu. SO 4 5 H 2 O (s) Cu. SO 4 (s) + 5 H 2 O (g) – N 2 (g) + 3 H 2 (g) 2 NH 3 (g)
![Values of K n Equilibrium constant, K, is found by: [products] [reactants] – If Values of K n Equilibrium constant, K, is found by: [products] [reactants] – If](http://slidetodoc.com/presentation_image_h2/03d483bae6611cc52fdeea70ea1d467b/image-16.jpg)
Values of K n Equilibrium constant, K, is found by: [products] [reactants] – If K = 1…. . • equal ratio of products and reactants – If K > 1 …. • reaction favors products – If K < 1 …. • reaction favors reactants
What changes K? 1. Change the temperature. – Equilibrium is temperature dependent.
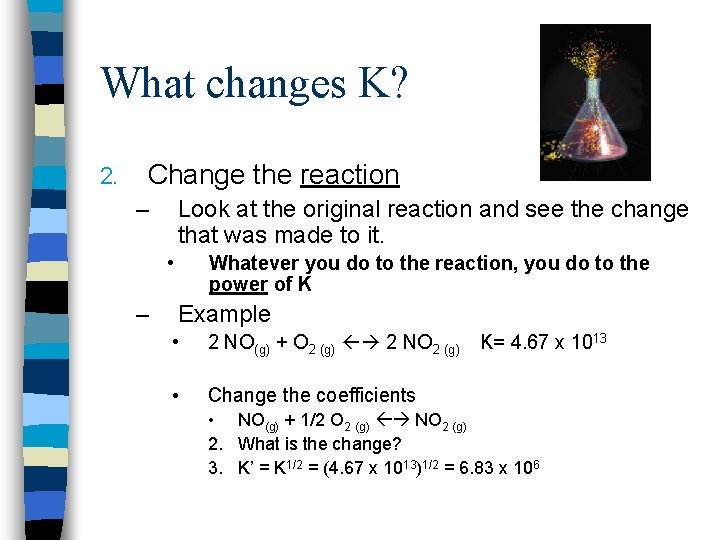
What changes K? 2. Change the reaction – Look at the original reaction and see the change that was made to it. • – Whatever you do to the reaction, you do to the power of K Example • 2 NO(g) + O 2 (g) 2 NO 2 (g) • Change the coefficients K= 4. 67 x 1013 • NO(g) + 1/2 O 2 (g) NO 2 (g) 2. What is the change? 3. K’ = K 1/2 = (4. 67 x 1013)1/2 = 6. 83 x 106
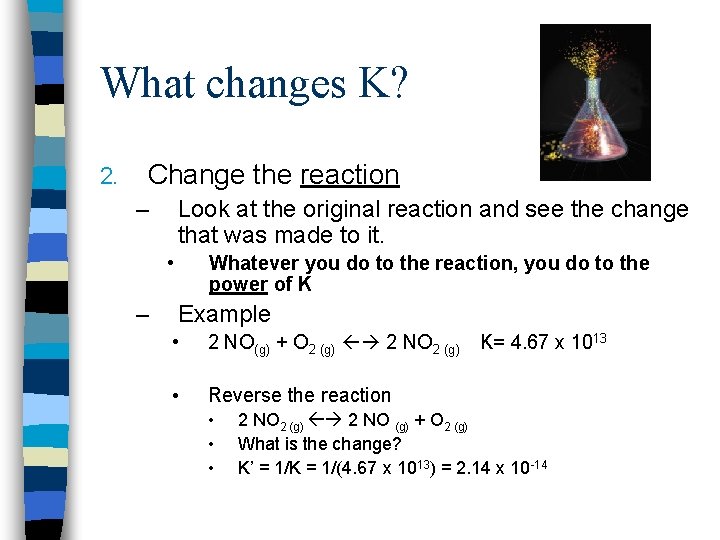
What changes K? 2. Change the reaction – Look at the original reaction and see the change that was made to it. • – Whatever you do to the reaction, you do to the power of K Example • 2 NO(g) + O 2 (g) 2 NO 2 (g) • Reverse the reaction • • • K= 4. 67 x 1013 2 NO 2 (g) 2 NO (g) + O 2 (g) What is the change? K’ = 1/K = 1/(4. 67 x 1013) = 2. 14 x 10 -14
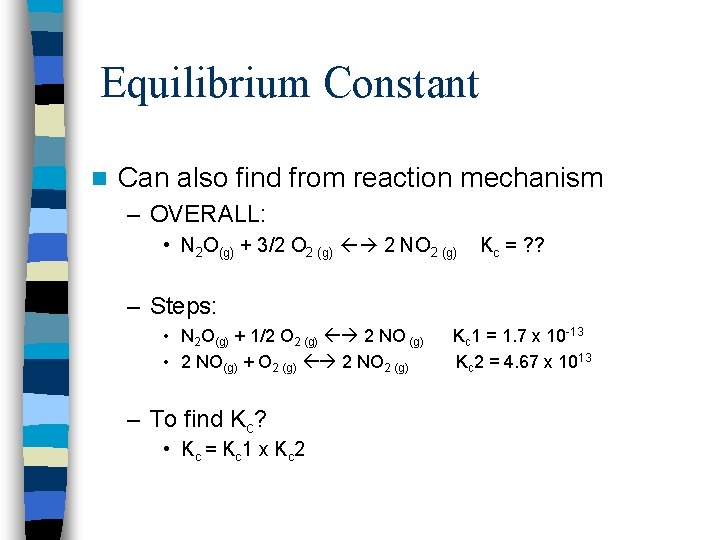
Equilibrium Constant n Can also find from reaction mechanism – OVERALL: • N 2 O(g) + 3/2 O 2 (g) 2 NO 2 (g) Kc = ? ? – Steps: • N 2 O(g) + 1/2 O 2 (g) 2 NO (g) • 2 NO(g) + O 2 (g) 2 NO 2 (g) – To find Kc? • Kc = Kc 1 x Kc 2 Kc 1 = 1. 7 x 10 -13 Kc 2 = 4. 67 x 1013
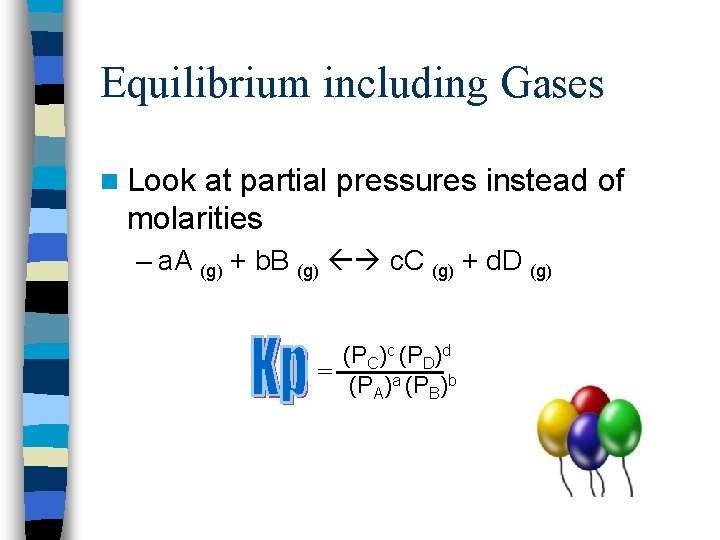
Equilibrium including Gases n Look at partial pressures instead of molarities – a. A (g) + b. B (g) c. C (g) + d. D (g) (PC)c (PD)d = (PA)a (PB)b
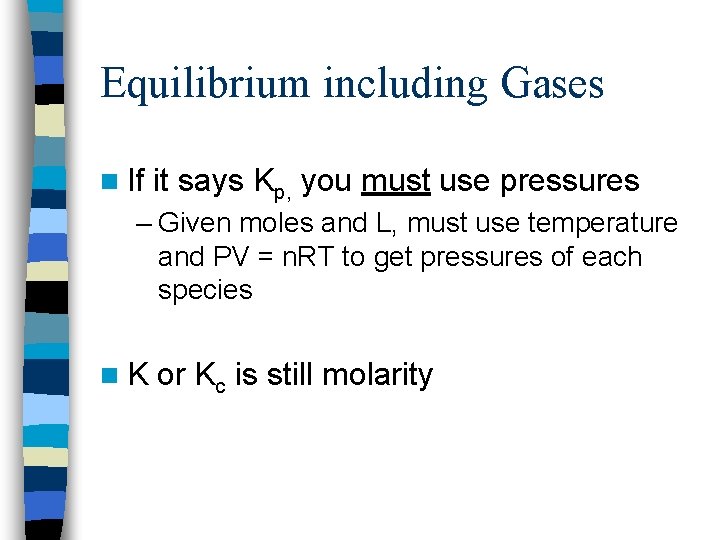
Equilibrium including Gases n If it says Kp, you must use pressures – Given moles and L, must use temperature and PV = n. RT to get pressures of each species n. K or Kc is still molarity
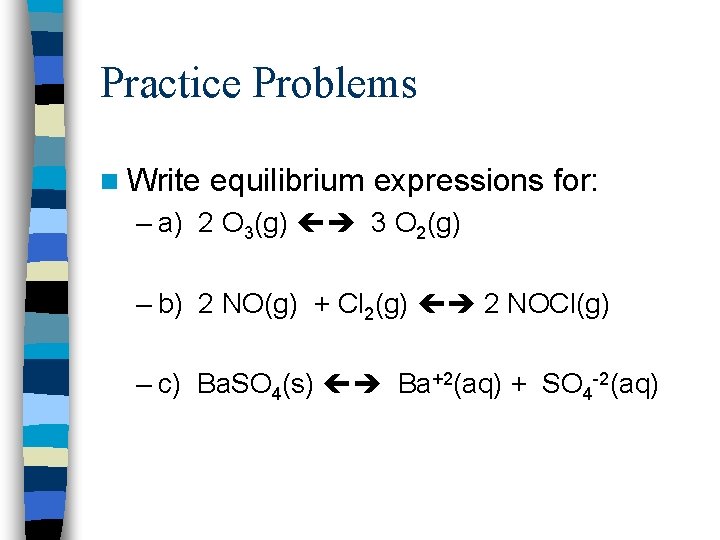
Practice Problems n Write equilibrium expressions for: – a) 2 O 3(g) 3 O 2(g) – b) 2 NO(g) + Cl 2(g) 2 NOCl(g) – c) Ba. SO 4(s) Ba+2(aq) + SO 4 -2(aq)
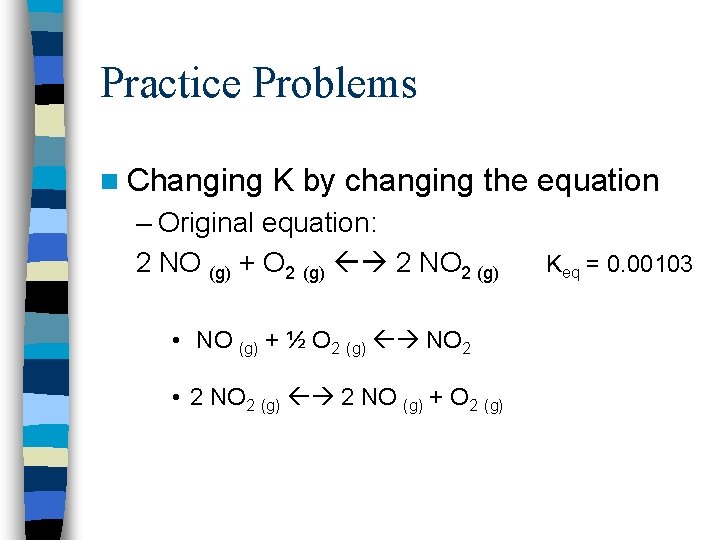
Practice Problems n Changing K by changing the equation – Original equation: 2 NO (g) + O 2 (g) 2 NO 2 (g) • NO (g) + ½ O 2 (g) NO 2 • 2 NO 2 (g) 2 NO (g) + O 2 (g) Keq = 0. 00103
- Slides: 24