Chemical Equilibrium The state where the concentrations of

Chemical Equilibrium The state where the concentrations of all reactants and products remain constant with time. n On the molecular level, there is frantic activity. Equilibrium is not static, but is a highly dynamic situation. n 1
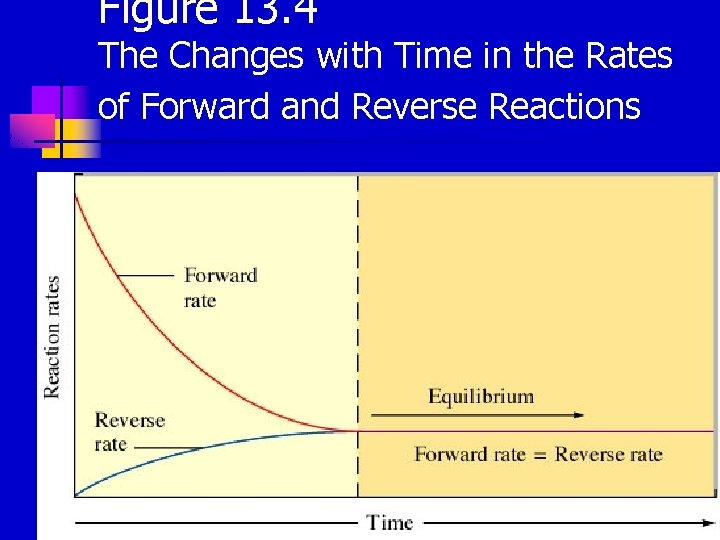
Figure 13. 4 The Changes with Time in the Rates of Forward and Reverse Reactions 2
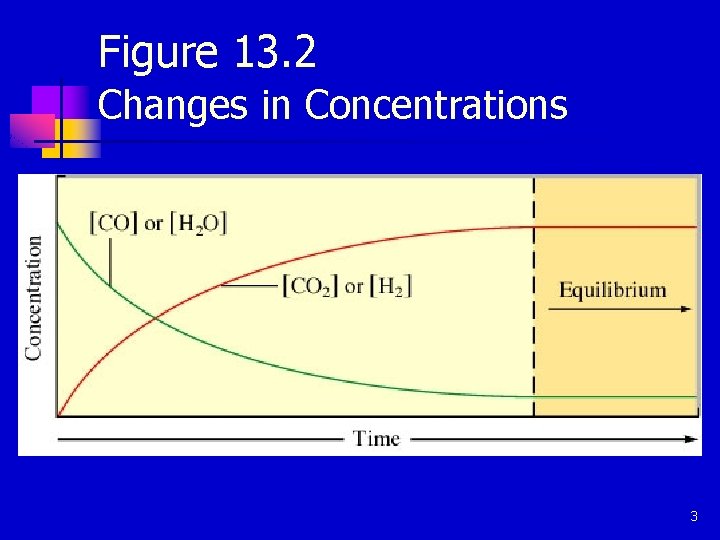
Figure 13. 2 Changes in Concentrations 3
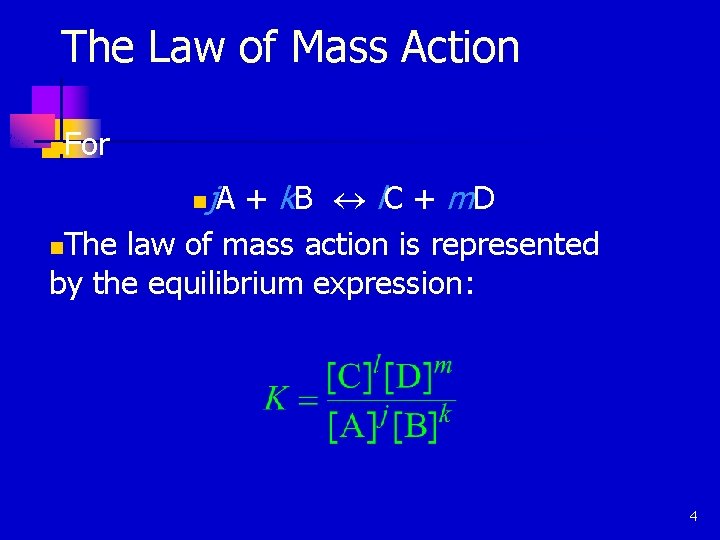
The Law of Mass Action n For n j A + k. B « l C + m D The law of mass action is represented by the equilibrium expression: n 4
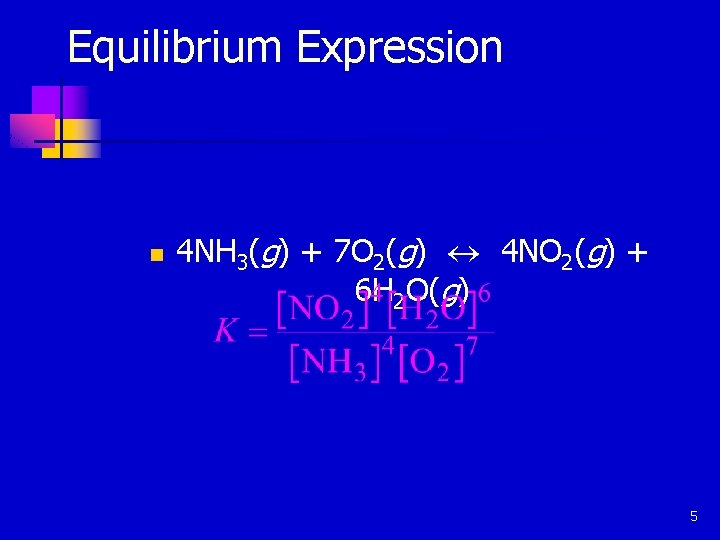
Equilibrium Expression n 4 NH 3(g) + 7 O 2(g) « 4 NO 2(g) + 6 H 2 O(g) 5

Notes on Equilibrium Expressions (EE) n n n The Equilibrium Expression for a reaction is the reciprocal of that for the reaction written in reverse. When the equation for a reaction is multiplied by n, EEnew = (EEoriginal)n The units for K are unimportant even though we use molarities or pressures. (We actually us affinities in higher level chem. which are unit less) 6
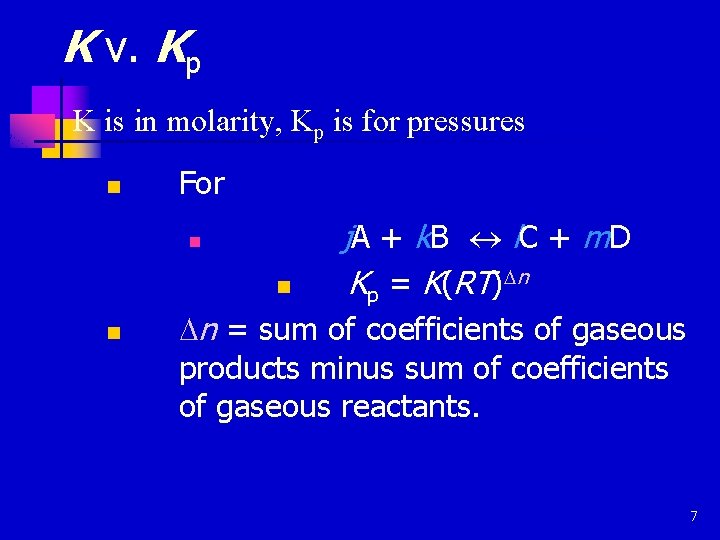
K v. Kp K is in molarity, Kp is for pressures n For n n n j A + k. B « l C + m D Kp = K(RT)Dn Dn = sum of coefficients of gaseous products minus sum of coefficients of gaseous reactants. 7
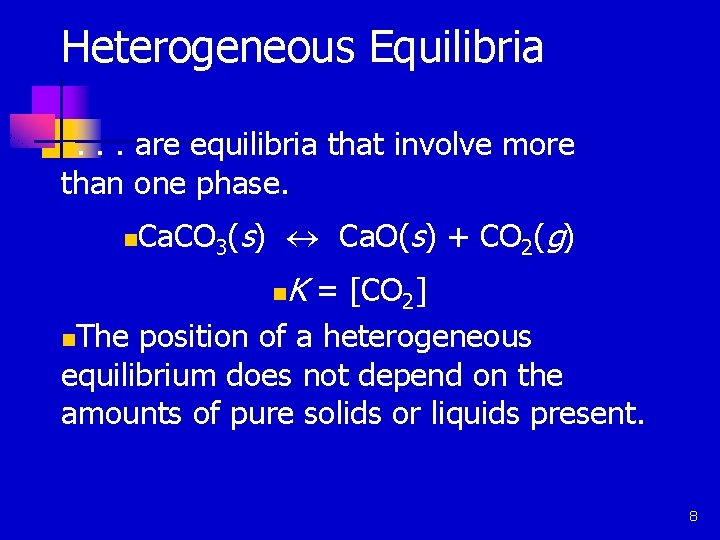
Heterogeneous Equilibria. . . are equilibria that involve more than one phase. n n Ca. CO 3(s) « Ca. O(s) + CO 2(g) n K = [CO 2] The position of a heterogeneous equilibrium does not depend on the amounts of pure solids or liquids present. n 8
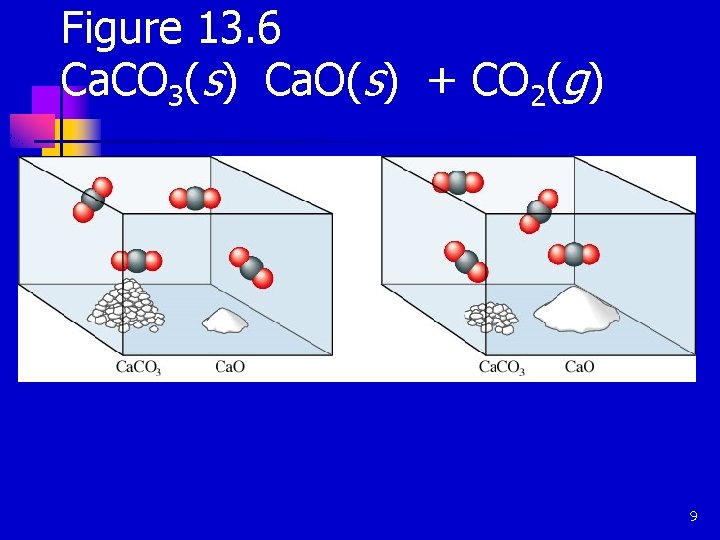
Figure 13. 6 Ca. CO 3(s) Ca. O(s) + CO 2(g) 9

Reaction Quotient. . . helps to determine the direction of the move toward equilibrium. n The law of mass action is applied with initial concentrations. n 10
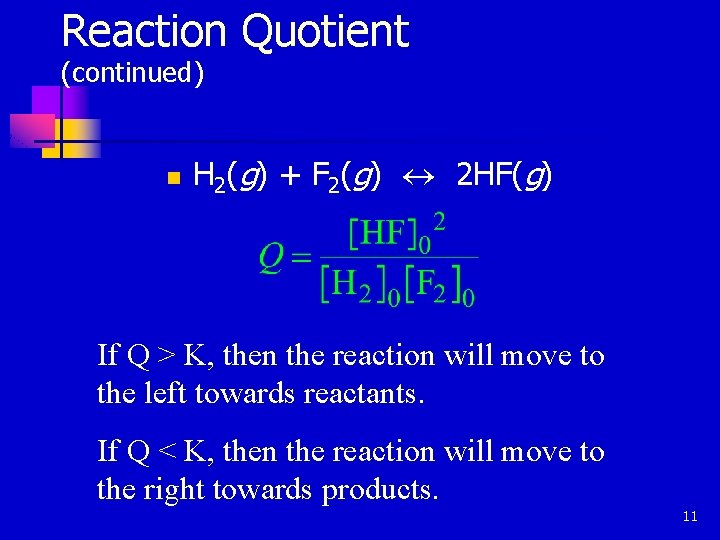
Reaction Quotient (continued) n H 2(g) + F 2(g) « 2 HF(g) If Q > K, then the reaction will move to the left towards reactants. If Q < K, then the reaction will move to the right towards products. 11
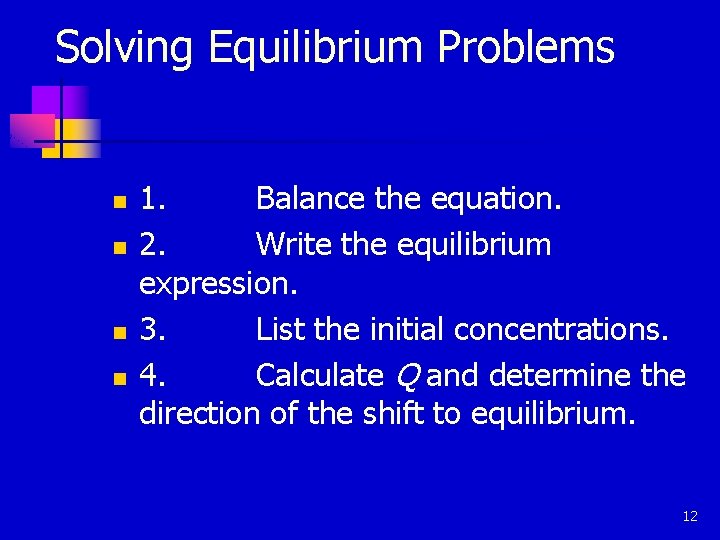
Solving Equilibrium Problems n n 1. Balance the equation. 2. Write the equilibrium expression. 3. List the initial concentrations. 4. Calculate Q and determine the direction of the shift to equilibrium. 12
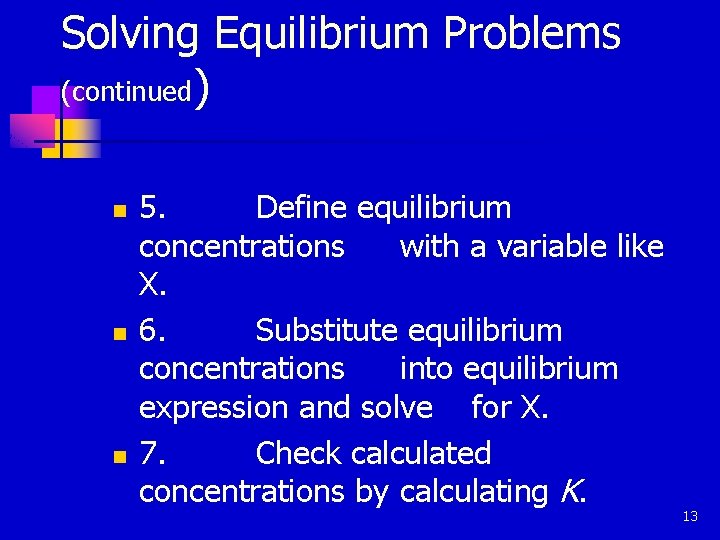
Solving Equilibrium Problems (continued) n n n 5. Define equilibrium concentrations with a variable like X. 6. Substitute equilibrium concentrations into equilibrium expression and solve for X. 7. Check calculated concentrations by calculating K. 13
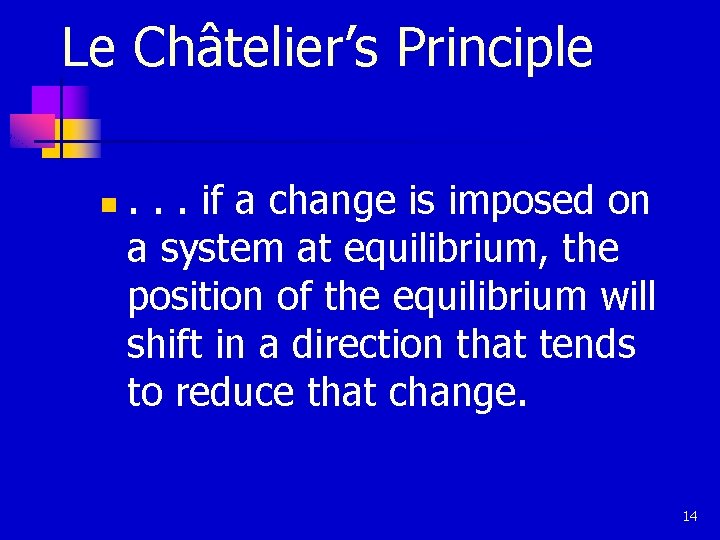
Le Châtelier’s Principle n . . . if a change is imposed on a system at equilibrium, the position of the equilibrium will shift in a direction that tends to reduce that change. 14
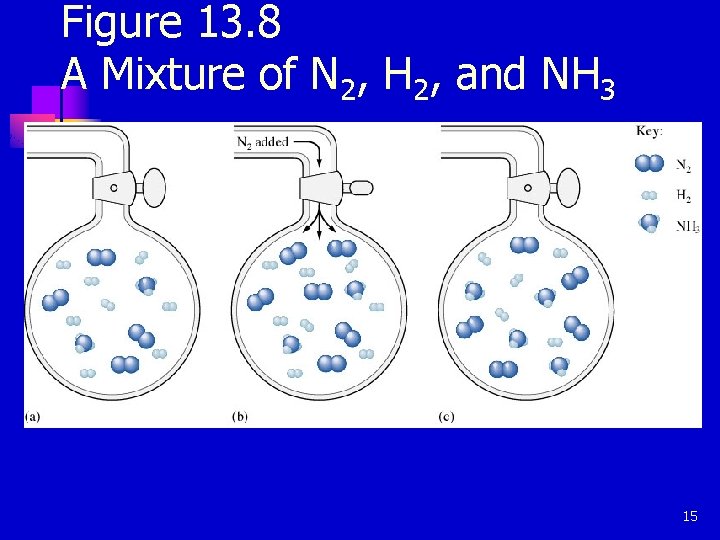
Figure 13. 8 A Mixture of N 2, H 2, and NH 3 15

Figure 13. 9 The Effect of Decreased Volume on the Ammonia Synthesis Equilibrium 16
Effects of Changes on the System n n 1. Concentration: The system will shift away from the added component. 2. Temperature: K will change depending upon the temperature (treat the energy change as a reactant). 17

Effects of Changes on the System (continued) n n n 3. Pressure: a. Addition of inert gas does not affect the equilibrium position. b. Decreasing the volume shifts the equilibrium toward the side with fewer moles. 18
- Slides: 18