Chemical Equilibrium The branch of chemistry which deals

Chemical Equilibrium

The branch of chemistry, which deals with the rate of chemical reactions or the factors affecting the rate of reactions and the mechanism of the reaction is called chemical kinetics. Rate of a chemical reaction is the change in the concentration of any one of the reactants or products per unit time. It is expressed in mol L-1 s-1 or Ms-1 or atm time-1 units. Rate of reaction = (decrease/increase in the concentration of reactant /product /time taken) This rate of reaction is known as average rate of reaction (rav). (rav can be calculated by dividing the concentration difference by the time interval).

Reversible and Irreversible change The reaction which can be reversed are termed as reversible reaction and the reaction which cannot be reversed are termed as irreversible reaction EQUILIBRIUM STATE When rate of formation of a product in a process is in competition with rate of formation of reactants, the state is then named as “Equilibrium state”. Equilibrium in physical processes: solid ⇌ liquid ⇌ gas H 2 O(s )⇌ H 2 O(l) ⇌ H 2 O(vap)
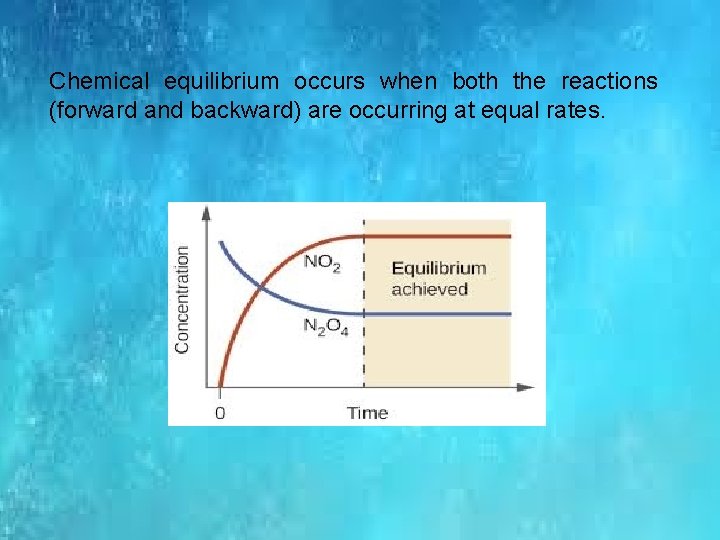
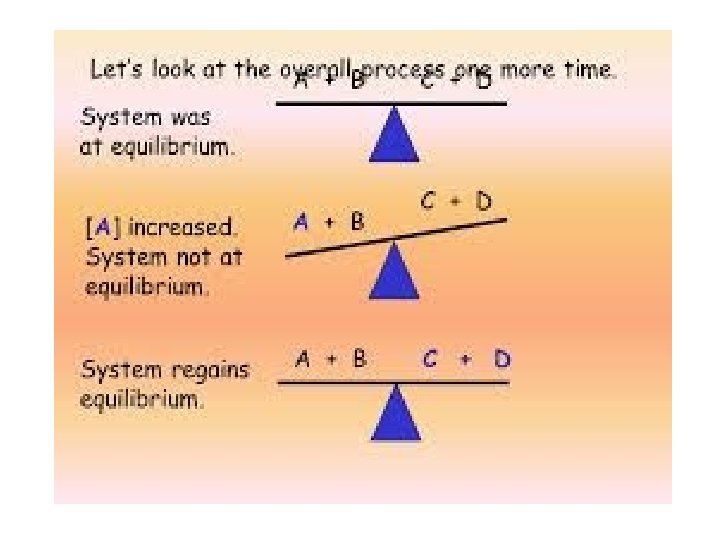
Chemical equilibrium occurs when both the reactions (forward and backward) are occurring at equal rates.

EQUILIBRIUM IN PHYSICAL PROCESS Some of the phase processes of equilibrium are as follows: • Solid-liquid equilibrium: For example, ice and water are equilibrium at only one temperature is called NORMAL MELTING POINT. • Liquid-gas equilibrium: For example, rate of evaporation = rate of condensation H 2 O(l) H 2 O(vap) • Solid-gas equilibrium: This process is also called as sublimation e. g. sublimation of camphor


Law of mass action At a given temperature the rate of reaction is directly proportional to molar concentration of reactants
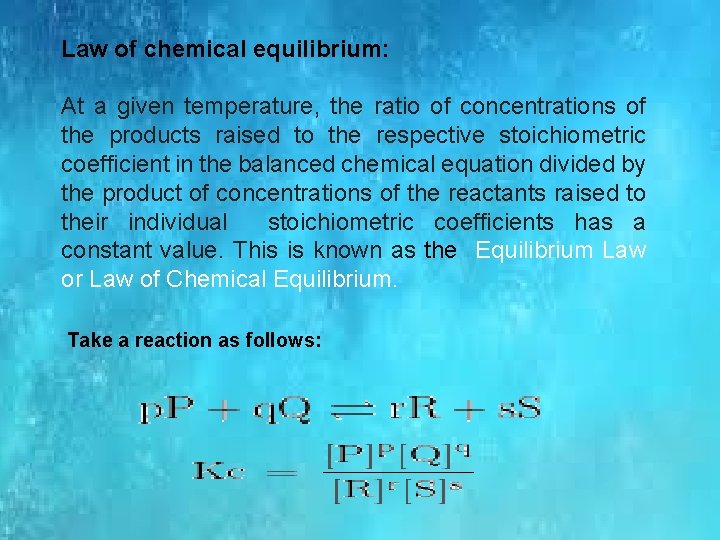
Law of chemical equilibrium: At a given temperature, the ratio of concentrations of the products raised to the respective stoichiometric coefficient in the balanced chemical equation divided by the product of concentrations of the reactants raised to their individual stoichiometric coefficients has a constant value. This is known as the Equilibrium Law or Law of Chemical Equilibrium. Take a reaction as follows:

Characteristics of K c 1. The reaction equilibrium can be obtained from both directions. 2. K c is a function of temperature. 3. K c is independent of the initial concentration of reactants and products. 4. If the K c value is • << 1, the equilibrium lies to the right and the reaction mixture contains mostly products. • 0. 10<K c<10, the mixture contains proportionate amount reactants and products. • >> 1, the equilibrium lies to the left and the reaction mixture contains mostly reactants.

Homogeneous Equilibrium When all the reactants and the products in the equilibrium are in the same phase, then this equilibrium is known as homogeneous equilibrium. For example: In this reaction, all these reactants are in the gaseous phase, thus it is homogeneous equilibrium
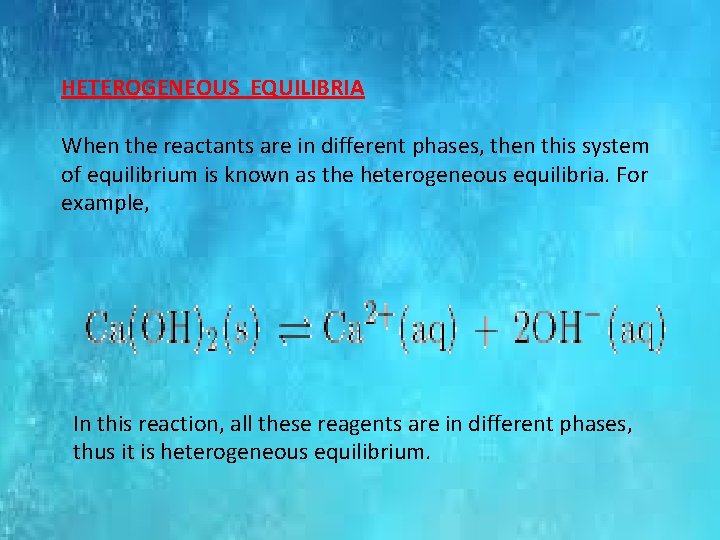
HETEROGENEOUS EQUILIBRIA When the reactants are in different phases, then this system of equilibrium is known as the heterogeneous equilibria. For example, In this reaction, all these reagents are in different phases, thus it is heterogeneous equilibrium.

Applications of Equilibrium Constant: • Predicts the extent of reaction, which gives the degree of the disappearance of reactants. • Predicts the direction of the reaction. • Calculating the equilibrium constant, which gives the relative amount of reactants and products

FACTORS CONSTANT. THAT AFFECT EQUILIBRIUM Temperature Pressure Catalyst: A catalyst does not affect equilibrium constant because it speeds up both forward and backward reactions to the same extent. Molar concentration of reactants and products.

RELATIONSHIP BETWEEN Kp AND Kc Kp is equilibrium constant in terms of partial pressure of gaseous reactants and products. Kc is equilibrium constant in terms of molar concentration of gaseous reactants and products. Kp =Kc (RT)∆n here R is gas constant, T is temperature at which the process is carried out &∆n is no. of moles of gaseous product minus no. of moles of gaseous reactants. If Kc> 103; Kc is very high i. e. the reaction proceeds nearly to completion. If Kc<103; Kc is very small i. e. the reaction proceeds rarely. If Kc is ranging in the range of 103 to 10 -3; i. e. reactants and products are just in equilibrium.

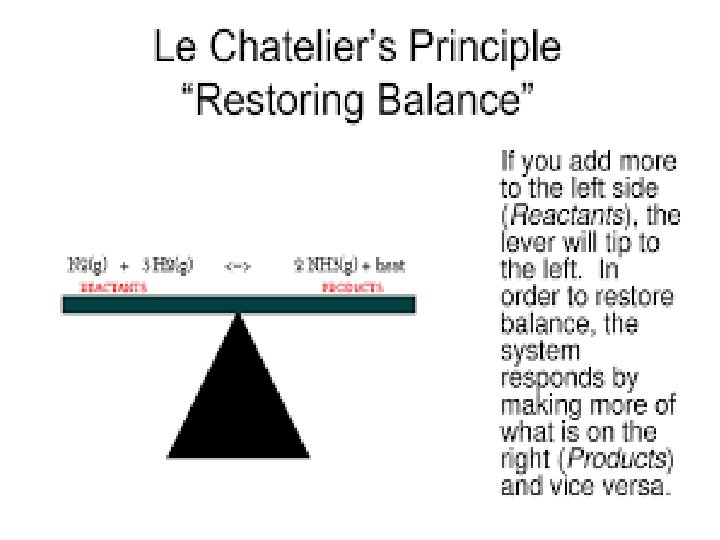
Le Chatelier’s principle: - It states that a change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counteract the effect of the change. Effect of Change of temperature Change of Pressure Change of volume according to Le Chatelier’s principle

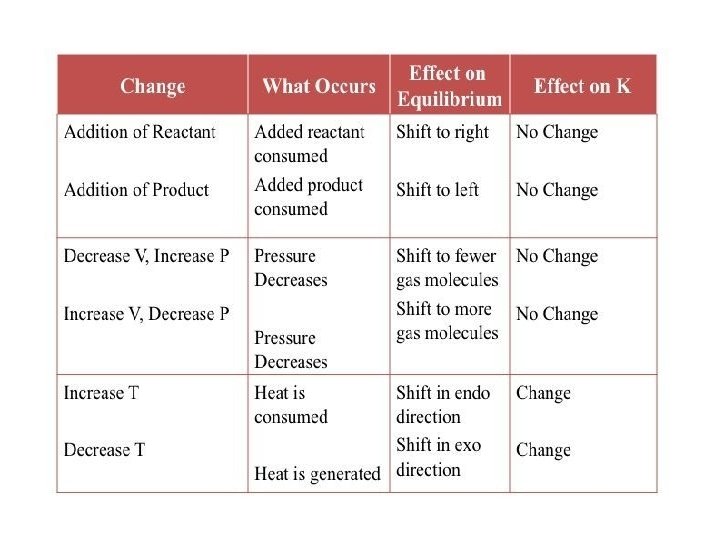
The Le–Chatelier’s. Principle: Effect of Change in Concentration: • When the concentration of reactants increased, equilibrium shifts in forward direction. • When the concentration of the products is increases, equilibrium shifts in backward direction. Effect of Change in Pressure: • Increase in pressure shifts the equilibrium in the direction of lesser number of gaseous molecules. • Decrease in pressure shifts the equilibrium in the direction of larger number of gaseous molecules.

Effect of Addition of Inert Gases: • Addition of inert gas at constant volume: No effect on equilibrium. • Addition of inert gas at constant pressure: Equilibrium shifts in a direction where there is increase in number of moles of gases. Effect of Change in Temperature: • In a system at equilibrium, both exothermic and endothermic reactions take place simultaneously. • Increase in temperature would shift the equilibrium in the direction of endothermic reaction. • Decrease in temperature would shift the equilibrium in the direction of exothermic reaction. Effect of Catalyst: Catalyst does not change the equilibrium
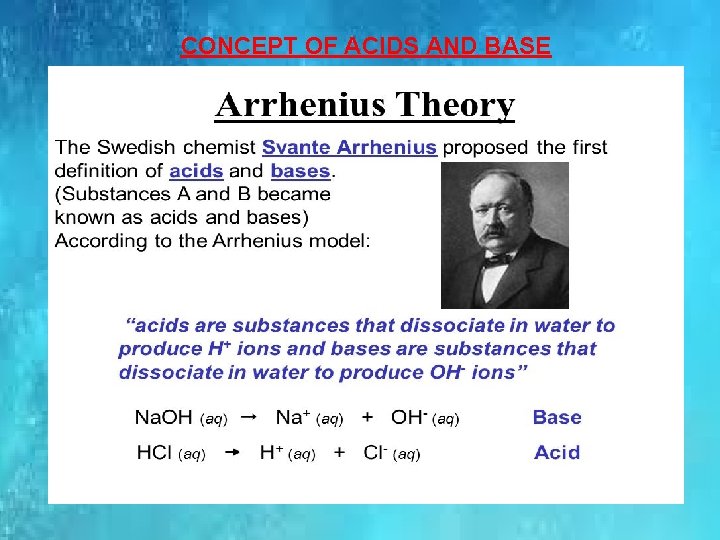
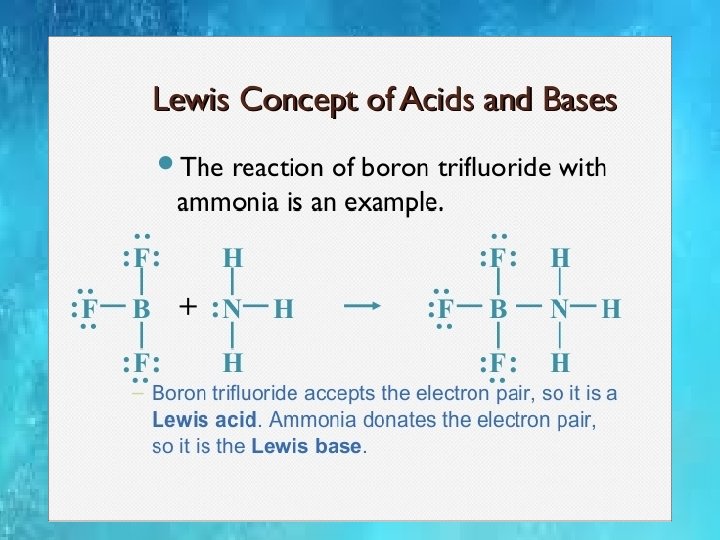
CONCEPT OF ACIDS AND BASE.
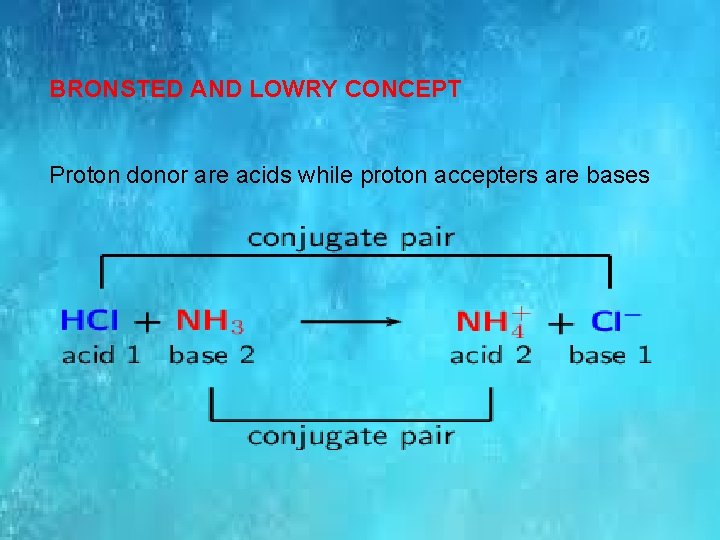
BRONSTED AND LOWRY CONCEPT Proton donor are acids while proton accepters are bases
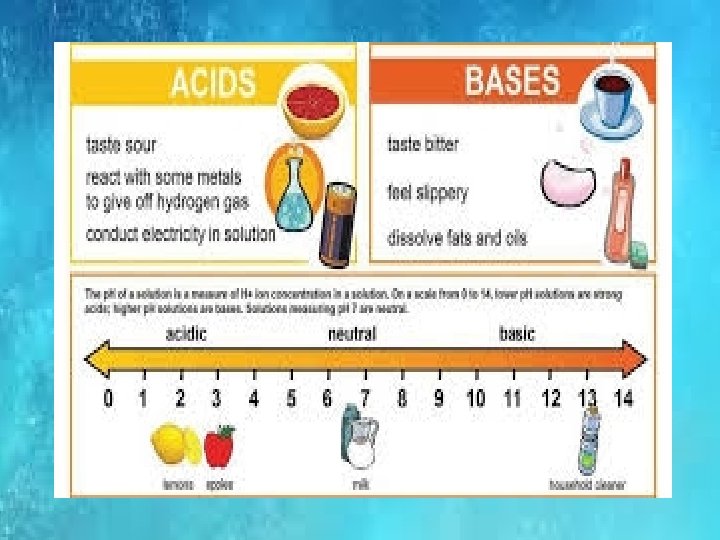
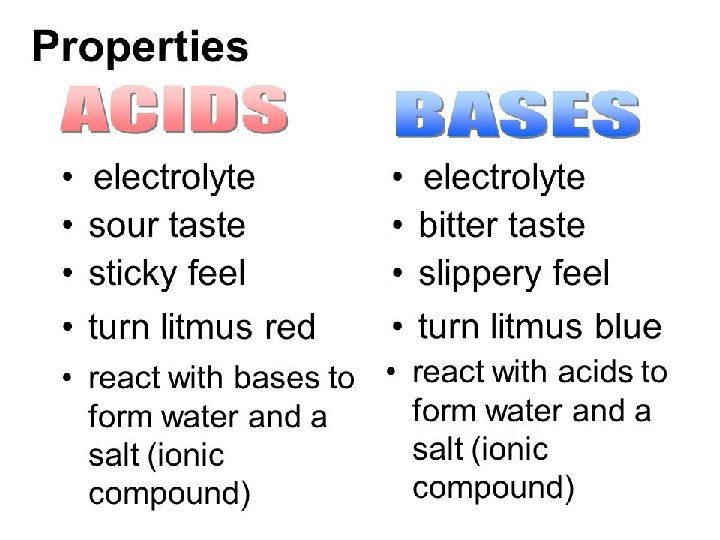
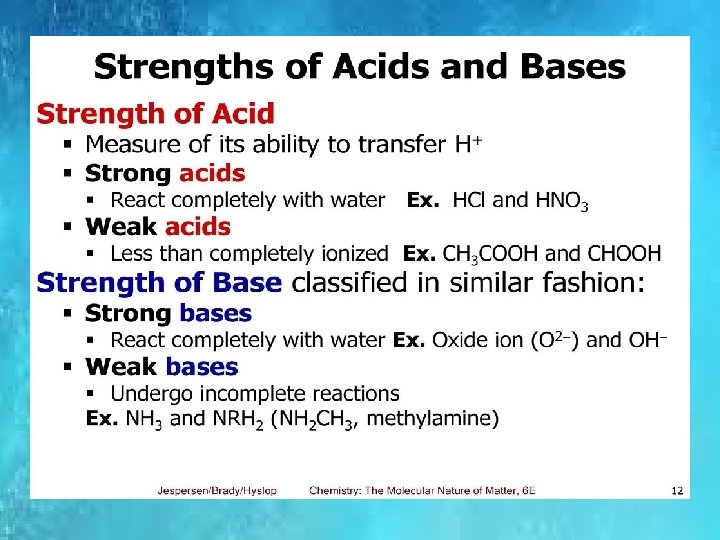
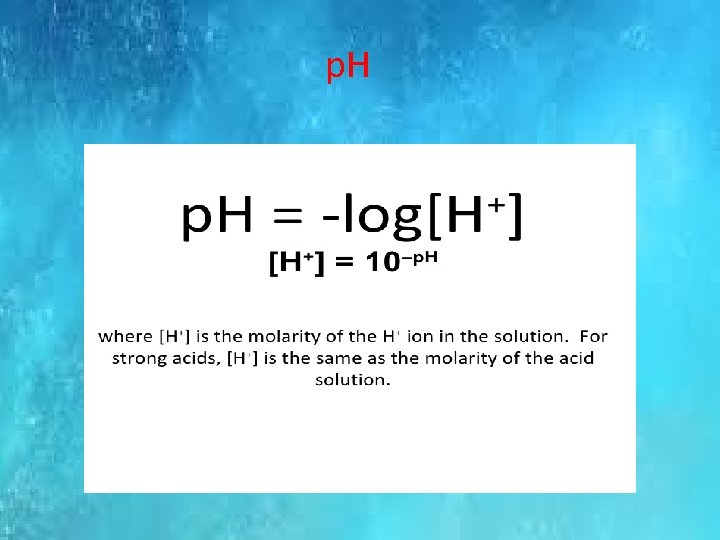
p. H
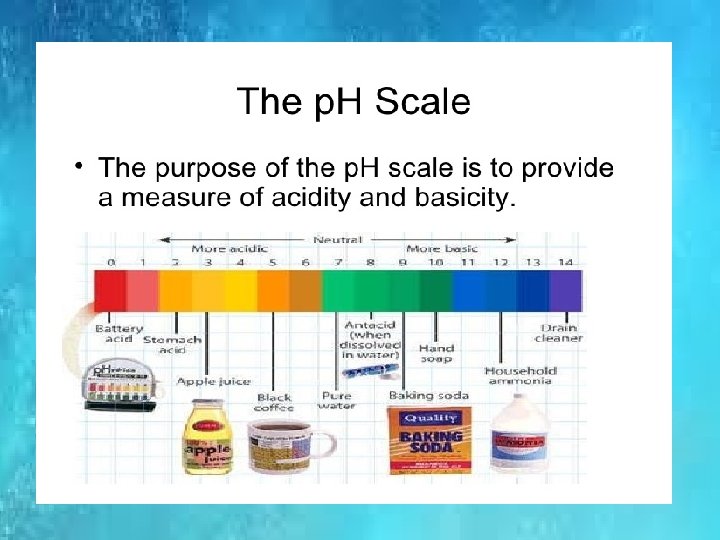
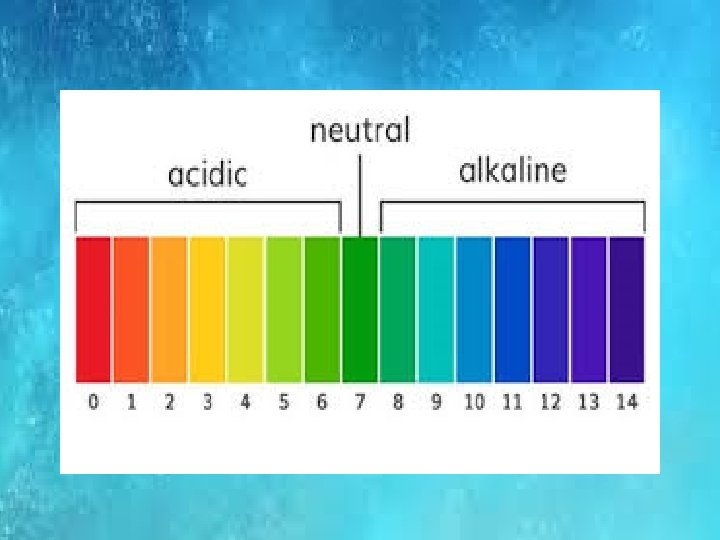
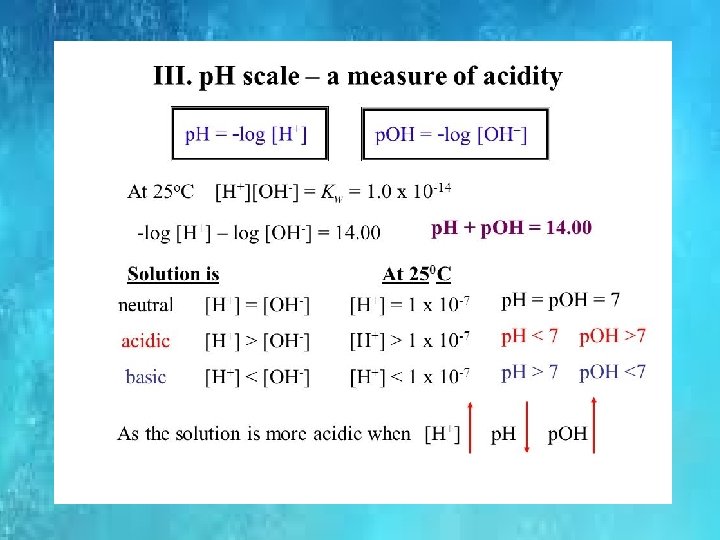
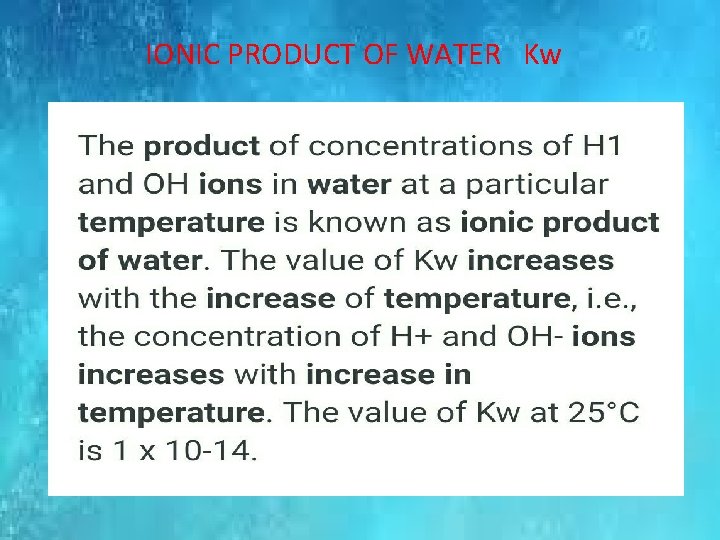
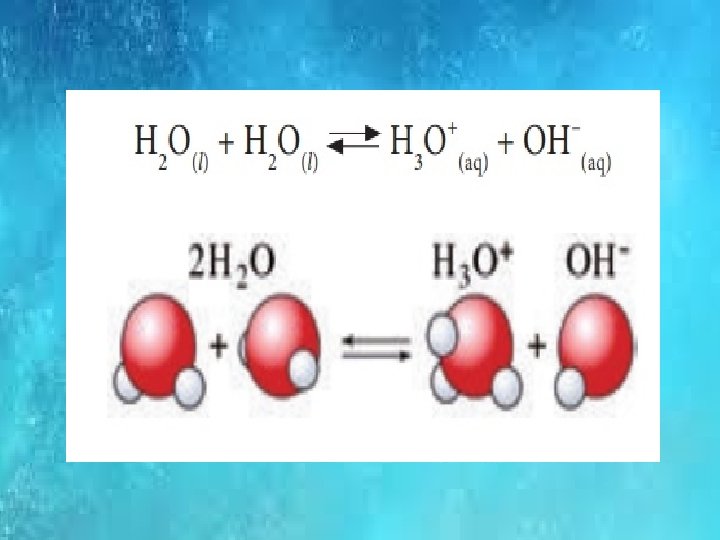
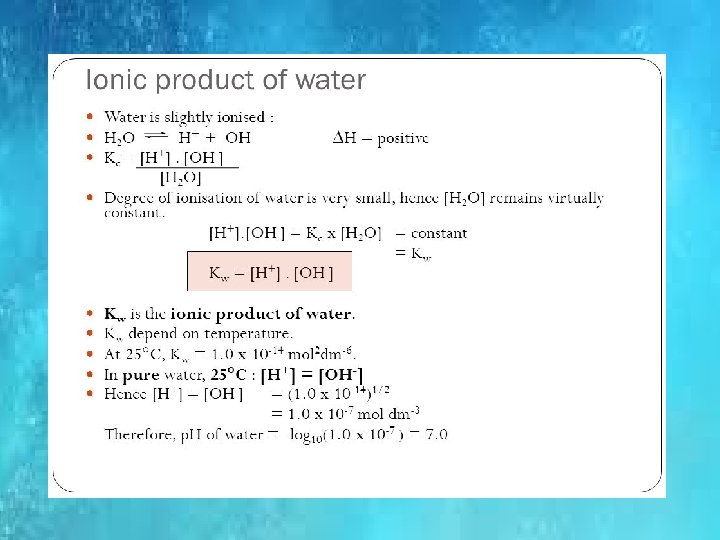
IONIC PRODUCT OF WATER Kw




- Slides: 39