Chemical Equilibrium Reversible and Irreversible Reactions Irreversible Reactions

Chemical Equilibrium

Reversible and Irreversible Reactions
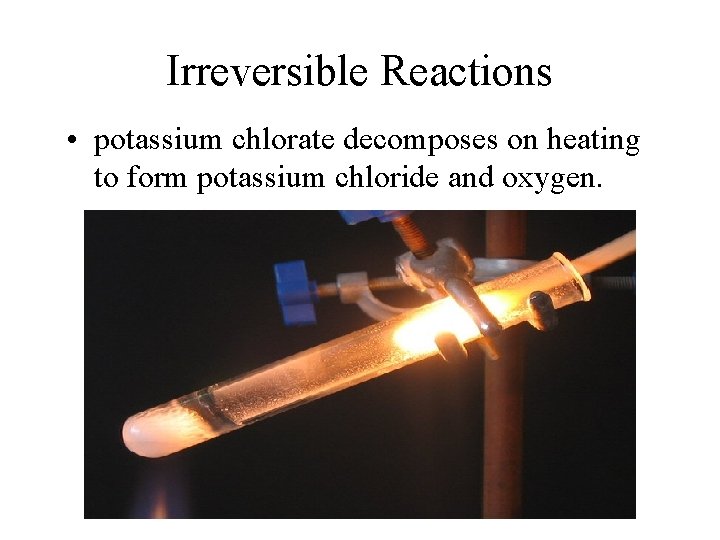
Irreversible Reactions • potassium chlorate decomposes on heating to form potassium chloride and oxygen.
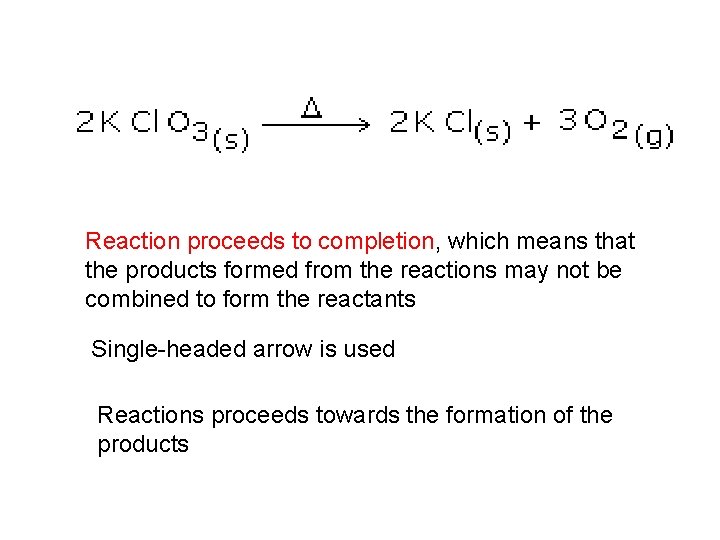
Reaction proceeds to completion, which means that the products formed from the reactions may not be combined to form the reactants Single-headed arrow is used Reactions proceeds towards the formation of the products

Reversible reactions A reversible reaction is a chemical change in which the products can be converted back to the original reactants under suitable conditions.
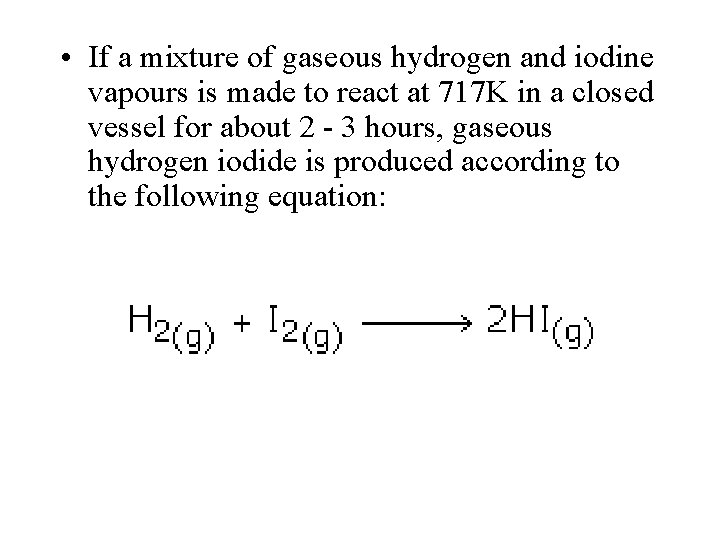
• If a mixture of gaseous hydrogen and iodine vapours is made to react at 717 K in a closed vessel for about 2 - 3 hours, gaseous hydrogen iodide is produced according to the following equation:
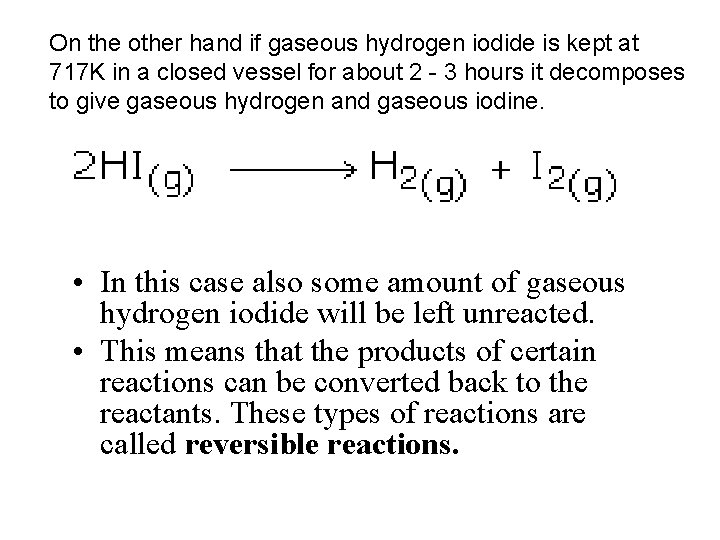
On the other hand if gaseous hydrogen iodide is kept at 717 K in a closed vessel for about 2 - 3 hours it decomposes to give gaseous hydrogen and gaseous iodine. • In this case also some amount of gaseous hydrogen iodide will be left unreacted. • This means that the products of certain reactions can be converted back to the reactants. These types of reactions are called reversible reactions.
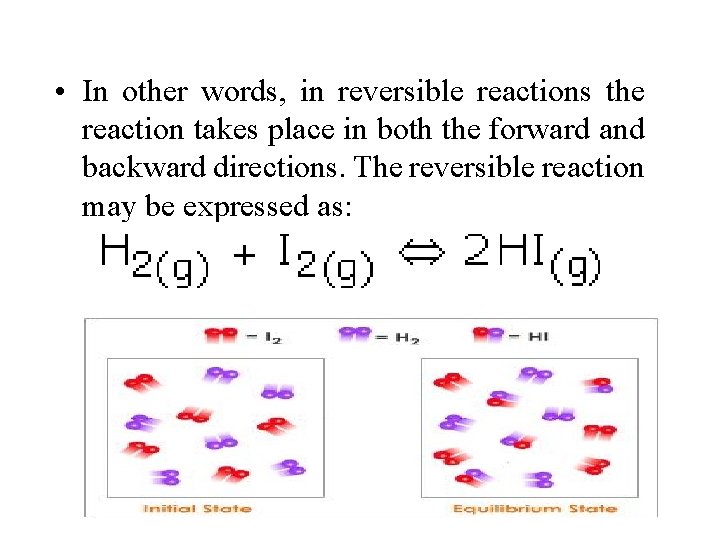
• In other words, in reversible reactions the reaction takes place in both the forward and backward directions. The reversible reaction may be expressed as:
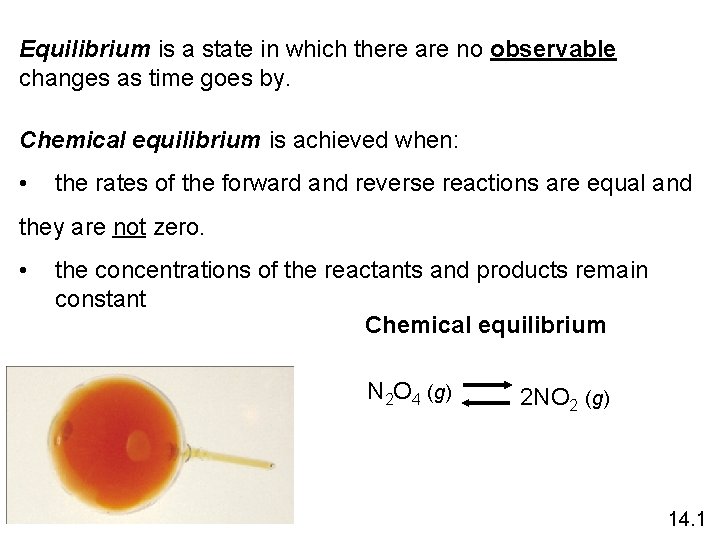
Equilibrium is a state in which there are no observable changes as time goes by. Chemical equilibrium is achieved when: • the rates of the forward and reverse reactions are equal and they are not zero. • the concentrations of the reactants and products remain constant Chemical equilibrium N 2 O 4 (g) 2 NO 2 (g) 14. 1

PHYSICAL EQUILIBRIUM Equilibrium between two phases of the same substance The changes that occur are physical processes Example : Vaporization of water in a closed container
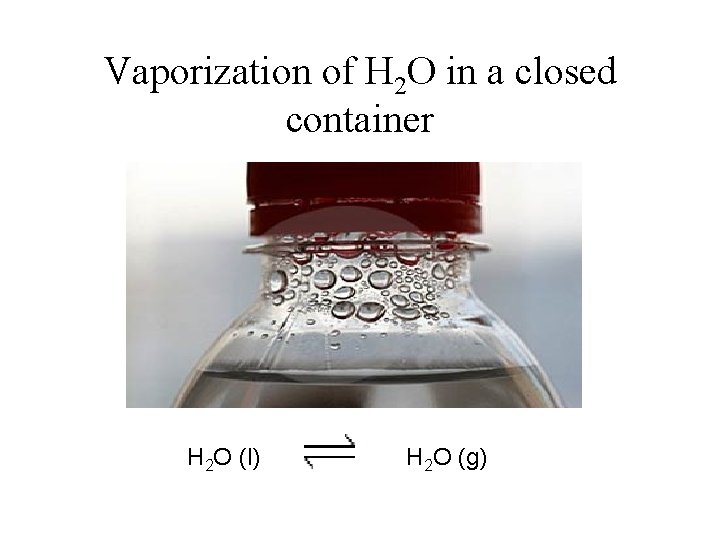
Vaporization of H 2 O in a closed container H 2 O (l) H 2 O (g)
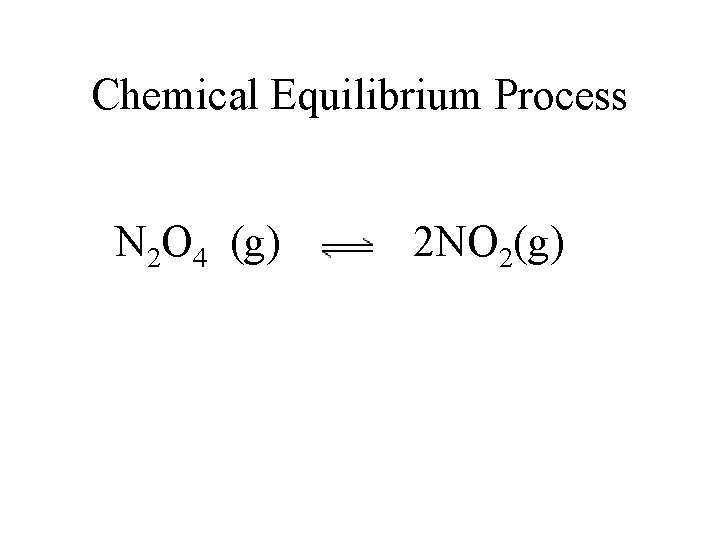
Chemical Equilibrium Process N 2 O 4 (g) 2 NO 2(g)
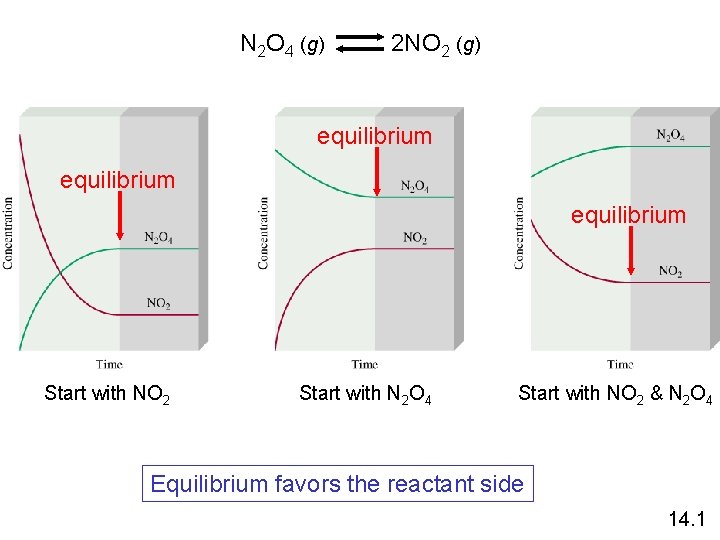
N 2 O 4 (g) 2 NO 2 (g) equilibrium Start with NO 2 Start with N 2 O 4 Start with NO 2 & N 2 O 4 Equilibrium favors the reactant side 14. 1
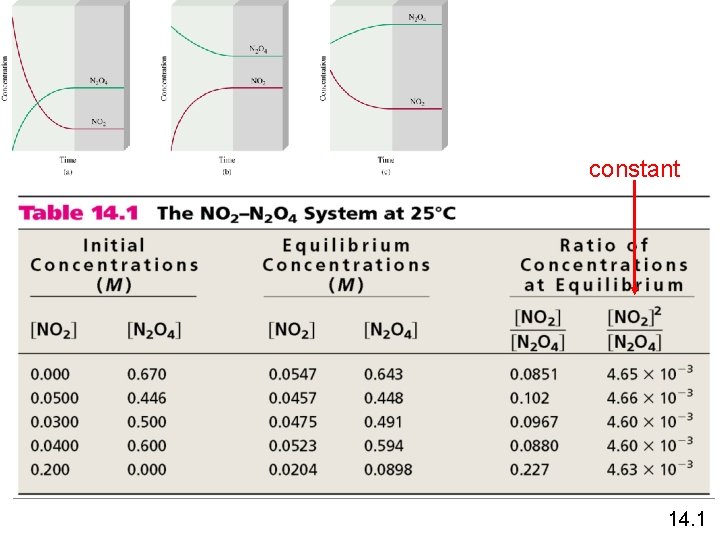
constant 14. 1
![N 2 O 4 (g) 2 NO 2 (g) [NO 2]2 = 4. 63 N 2 O 4 (g) 2 NO 2 (g) [NO 2]2 = 4. 63](http://slidetodoc.com/presentation_image_h2/7627e02ac3f1ed3ff5311e09ed2cdb78/image-15.jpg)
N 2 O 4 (g) 2 NO 2 (g) [NO 2]2 = 4. 63 x 10 -3 Kc = [N 2 O 4] a. A + b. B c. C + d. D [C]c[D]d K c= a b [A] [B] Equilibrium Will K >> 1 Lie to the right Favor products K << 1 Lie to the left Favor reactants 14. 1
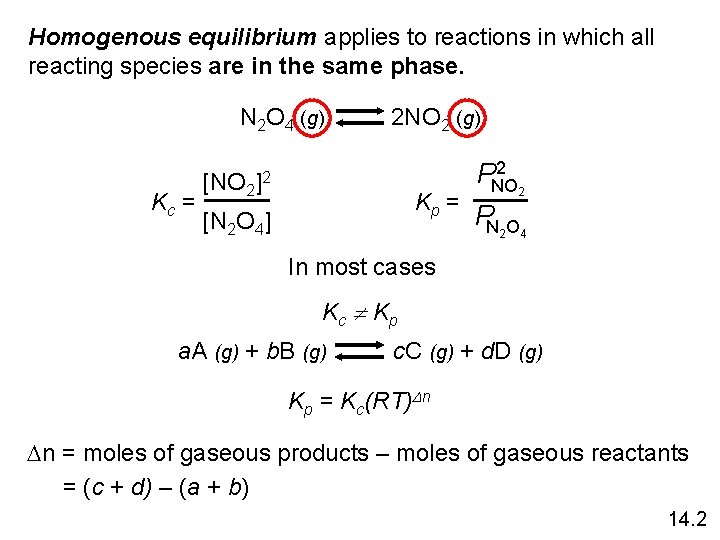
Homogenous equilibrium applies to reactions in which all reacting species are in the same phase. N 2 O 4 (g) Kc = [NO 2 2 NO 2 (g) ]2 Kp = [N 2 O 4] 2 PNO 2 PN 2 O 4 In most cases Kc K p a. A (g) + b. B (g) c. C (g) + d. D (g) Kp = Kc(RT)Dn Dn = moles of gaseous products – moles of gaseous reactants = (c + d) – (a + b) 14. 2
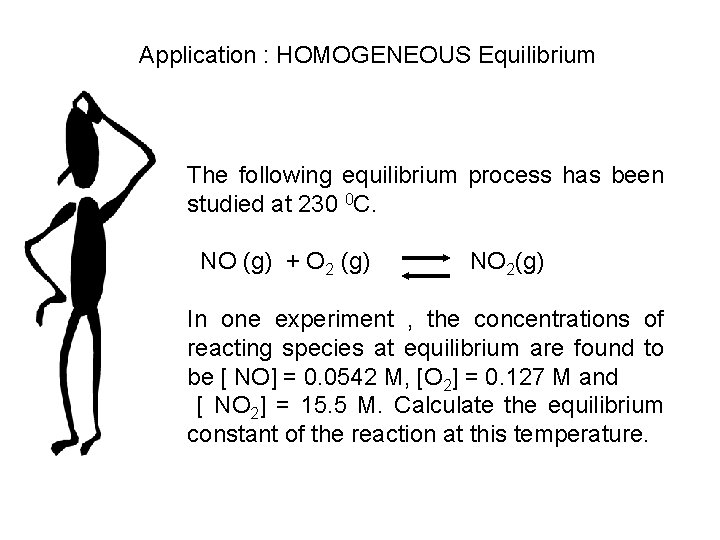
Application : HOMOGENEOUS Equilibrium The following equilibrium process has been studied at 230 0 C. NO (g) + O 2 (g) NO 2(g) In one experiment , the concentrations of reacting species at equilibrium are found to be [ NO] = 0. 0542 M, [O 2] = 0. 127 M and [ NO 2] = 15. 5 M. Calculate the equilibrium constant of the reaction at this temperature.
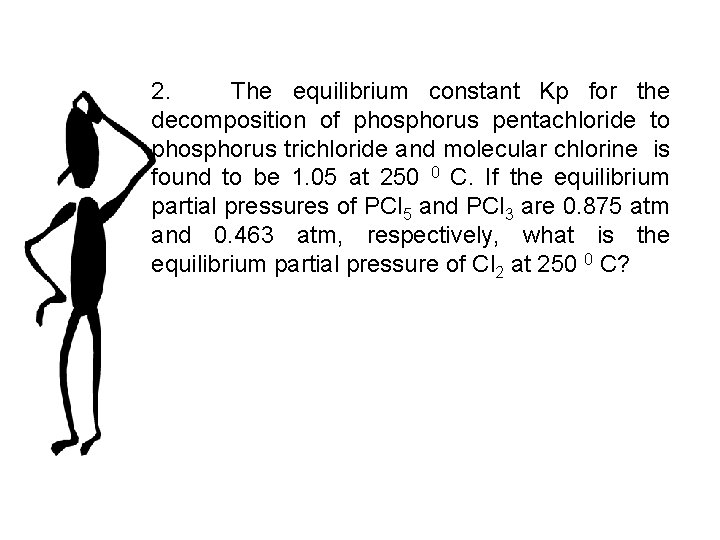
2. The equilibrium constant Kp for the decomposition of phosphorus pentachloride to phosphorus trichloride and molecular chlorine is found to be 1. 05 at 250 0 C. If the equilibrium partial pressures of PCl 5 and PCl 3 are 0. 875 atm and 0. 463 atm, respectively, what is the equilibrium partial pressure of Cl 2 at 250 0 C?
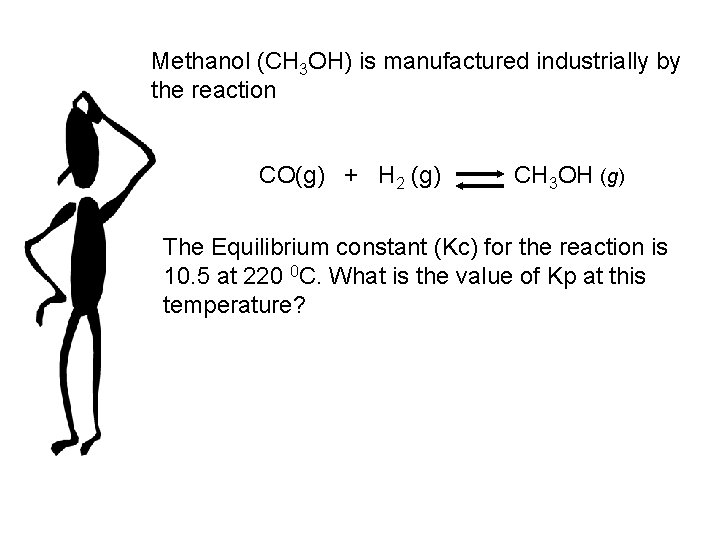
Methanol (CH 3 OH) is manufactured industrially by the reaction CO(g) + H 2 (g) CH 3 OH (g) The Equilibrium constant (Kc) for the reaction is 10. 5 at 220 0 C. What is the value of Kp at this temperature?
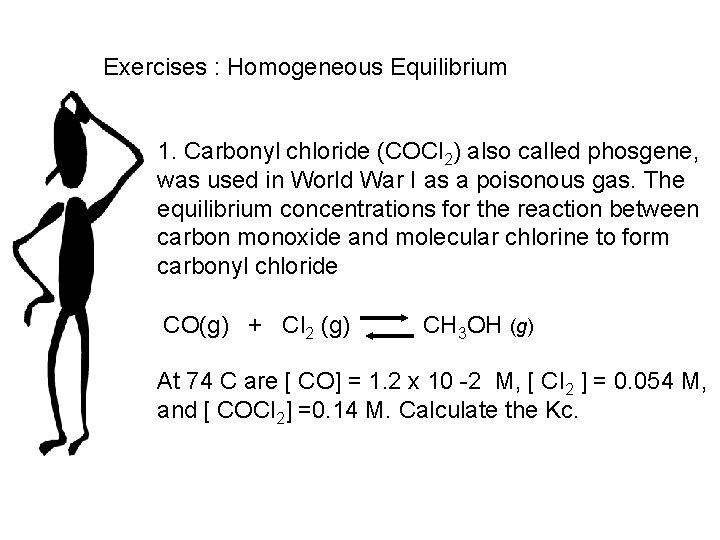
Exercises : Homogeneous Equilibrium 1. Carbonyl chloride (COCl 2) also called phosgene, was used in World War I as a poisonous gas. The equilibrium concentrations for the reaction between carbon monoxide and molecular chlorine to form carbonyl chloride CO(g) + Cl 2 (g) CH 3 OH (g) At 74 C are [ CO] = 1. 2 x 10 -2 M, [ Cl 2 ] = 0. 054 M, and [ COCl 2] =0. 14 M. Calculate the Kc.
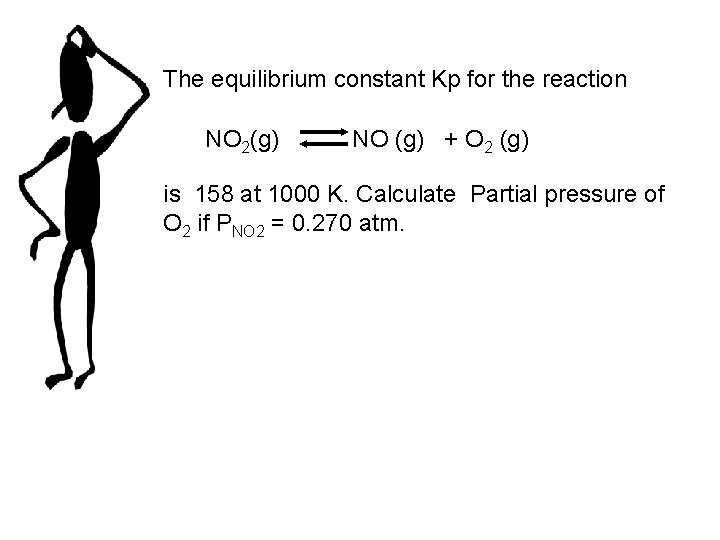
The equilibrium constant Kp for the reaction NO 2(g) NO (g) + O 2 (g) is 158 at 1000 K. Calculate Partial pressure of O 2 if PNO 2 = 0. 270 atm.
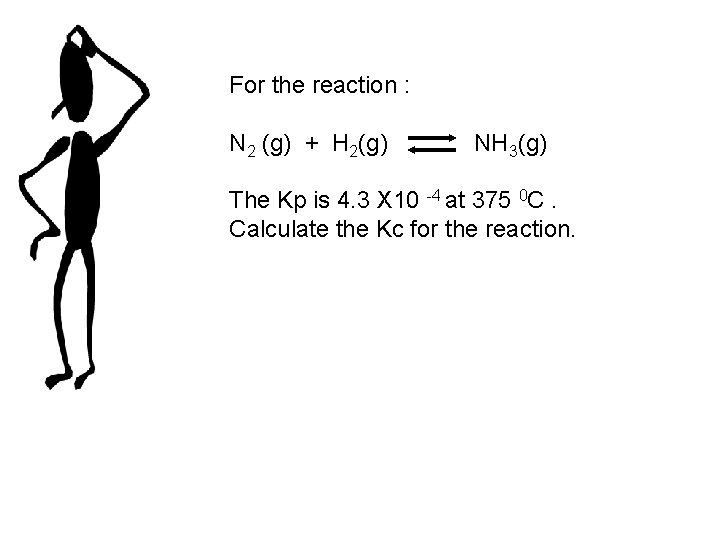
For the reaction : N 2 (g) + H 2(g) NH 3(g) The Kp is 4. 3 X 10 -4 at 375 0 C. Calculate the Kc for the reaction.
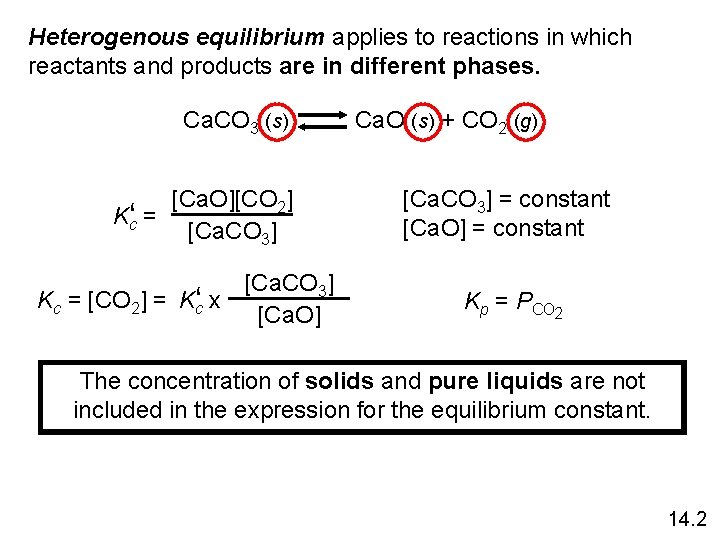
Heterogenous equilibrium applies to reactions in which reactants and products are in different phases. Ca. CO 3 (s) [Ca. O][CO 2] Kc‘ = [Ca. CO 3] Kc = [CO 2] = Kc‘ x [Ca. O] Ca. O (s) + CO 2 (g) [Ca. CO 3] = constant [Ca. O] = constant Kp = PCO 2 The concentration of solids and pure liquids are not included in the expression for the equilibrium constant. 14. 2
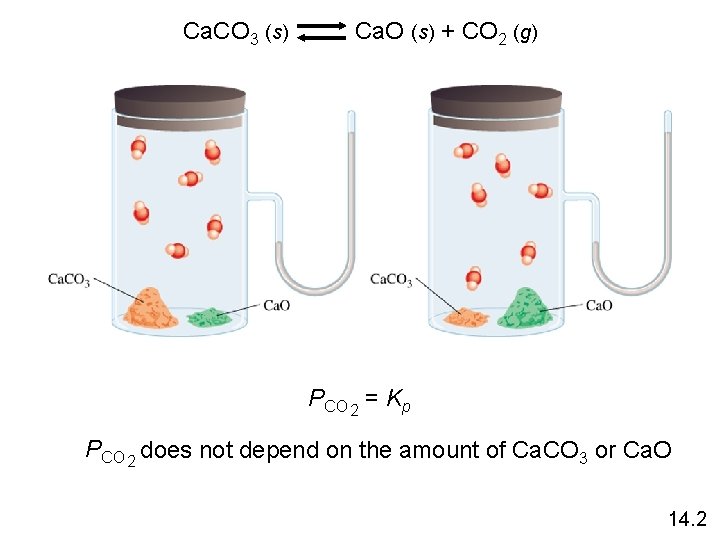
Ca. CO 3 (s) Ca. O (s) + CO 2 (g) PCO 2 = Kp PCO 2 does not depend on the amount of Ca. CO 3 or Ca. O 14. 2

Writing Equilibrium Constant Expressions • The concentrations of the reacting species in the condensed phase are expressed in M. In the gaseous phase, the concentrations can be expressed in M or in atm. • The concentrations of pure solids, pure liquids and solvents do not appear in the equilibrium constant expressions. • The equilibrium constant is a dimensionless quantity. • In quoting a value for the equilibrium constant, you must specify the balanced equation and the temperature. 14. 2

Application : Multiple Equilibria
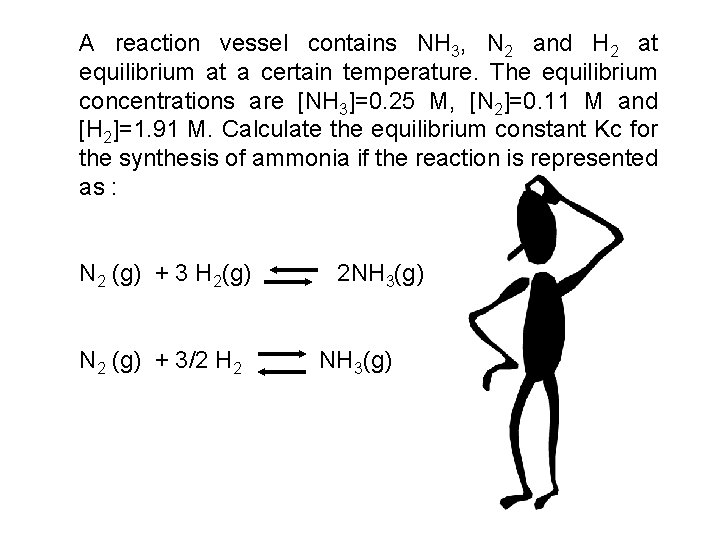
A reaction vessel contains NH 3, N 2 and H 2 at equilibrium at a certain temperature. The equilibrium concentrations are [NH 3]=0. 25 M, [N 2]=0. 11 M and [H 2]=1. 91 M. Calculate the equilibrium constant Kc for the synthesis of ammonia if the reaction is represented as : N 2 (g) + 3 H 2(g) N 2 (g) + 3/2 H 2 2 NH 3(g)

What Does the Equilibrium Constant Tell Us? It helps us to predict the direction in which the a reaction mixture will proceed to achieve equilibrium and to calculate the concentrations of reactants and products once equilibrium has been reached.
Predicting Reaction Direction from a Nonequilibrium Mixture • • Calculate Reaction Quotient, Qc Reaction Quotient(Qc)= Initial Conc. Of Products/ Initial Conc. of Reactants
• • The expression for Q is the same as K BUT nonequilibrium concentrations rather than equilibrium concentrations are put into the expression Compare the value of Q to K.
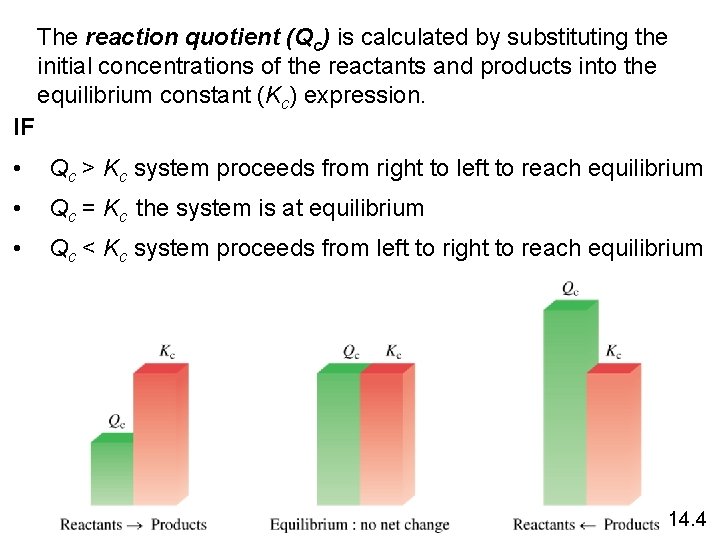
The reaction quotient (Qc) is calculated by substituting the initial concentrations of the reactants and products into the equilibrium constant (Kc) expression. IF • Qc > Kc system proceeds from right to left to reach equilibrium • Qc = Kc the system is at equilibrium • Qc < Kc system proceeds from left to right to reach equilibrium 14. 4
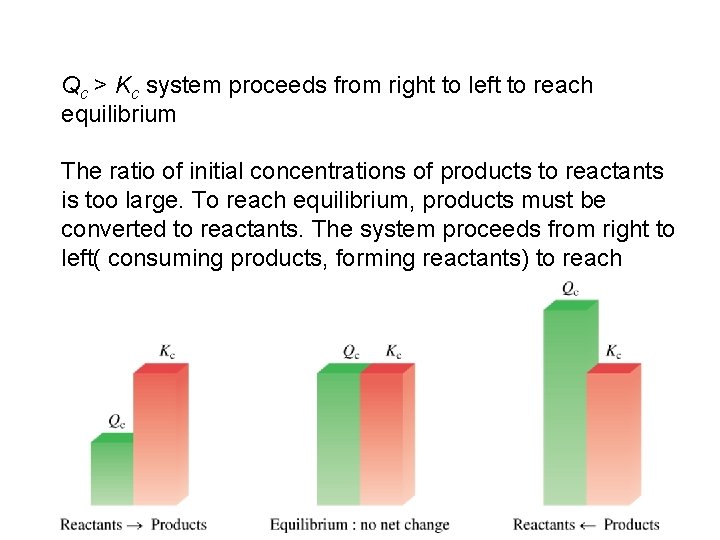
Qc > Kc system proceeds from right to left to reach equilibrium The ratio of initial concentrations of products to reactants is too large. To reach equilibrium, products must be converted to reactants. The system proceeds from right to left( consuming products, forming reactants) to reach equilibrium
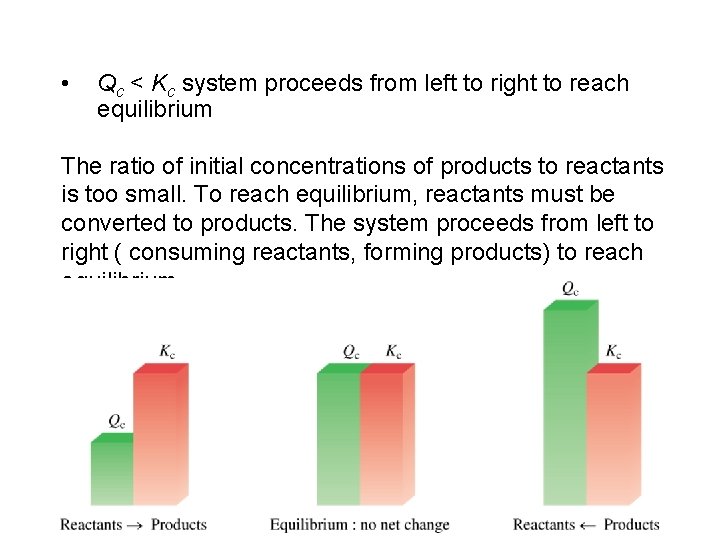
• Qc < Kc system proceeds from left to right to reach equilibrium The ratio of initial concentrations of products to reactants is too small. To reach equilibrium, reactants must be converted to products. The system proceeds from left to right ( consuming reactants, forming products) to reach equilibrium
Example • The equilibrium constant , Kc for the formation of Hydrogen iodide from molecular hydrogen and molecular Iodine in the gas phase is 54. 3 at 430 °C. Suppose in a certain experiment we place 0. 243 mole of H 2, 0. 146 mole of I 2 and 1. 98 moles of HI all in a 1. 00 L at 430 °C. Will there be a net reaction to form more H 2 and I 2 or more HI ?
Example 2 • At a start of reaction, there are 0. 249 mol N 2, 3. 21 x 10 -2 mol H 2, and 6. 42 x 10 -4 mol NH 3 in a 3. 50 -L reaction vessel at 375 °C. If the equilibrium constant(Kc) for the reaction is 1. 2 at this temperature, decide whether the system is at equilibrium. If it is not, predict which way the net reaction will proceed.

Calculating Equilibrium Concentrations • Consider the following system involving two organic compounds, cis-stilbene and trans- stilbene, in a non polar hydrocarbon solvent. The Kc for this system is 24. 0 at 200 °C. Suppose that initially only cisstilbene is present at a concentration of 0. 850 mol/L. How do we calculate the concentrations of cisstibene and trans stilbene at equilibrium?
• A mixture of 0. 500 mol of H 2 and 0. 500 mol of I 2 was placed in a 1. 00 -L stainless –steel flask at 430 C. The equilibrium constant for the reaction H 2(g) + I 2(g) 2 HI(g) is 54. 3 at this temperature. Calculate the concentrations of H 2, I 2 and HI equilibrium.

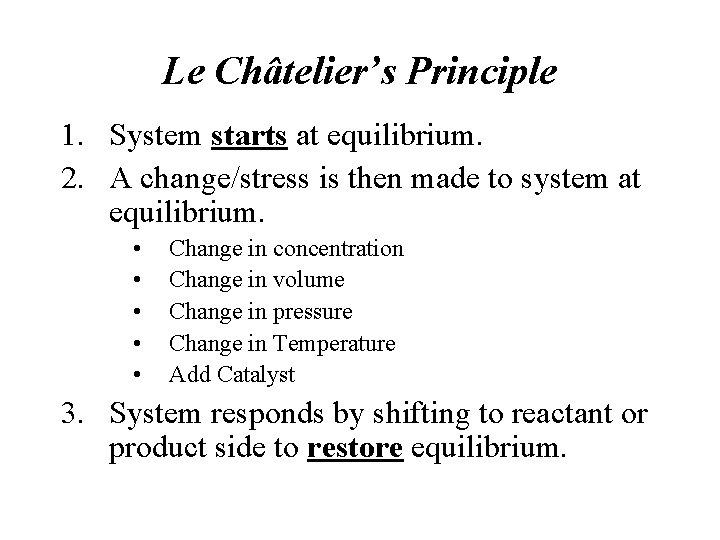
Le Châtelier’s Principle 1. System starts at equilibrium. 2. A change/stress is then made to system at equilibrium. • • • Change in concentration Change in volume Change in pressure Change in Temperature Add Catalyst 3. System responds by shifting to reactant or product side to restore equilibrium.
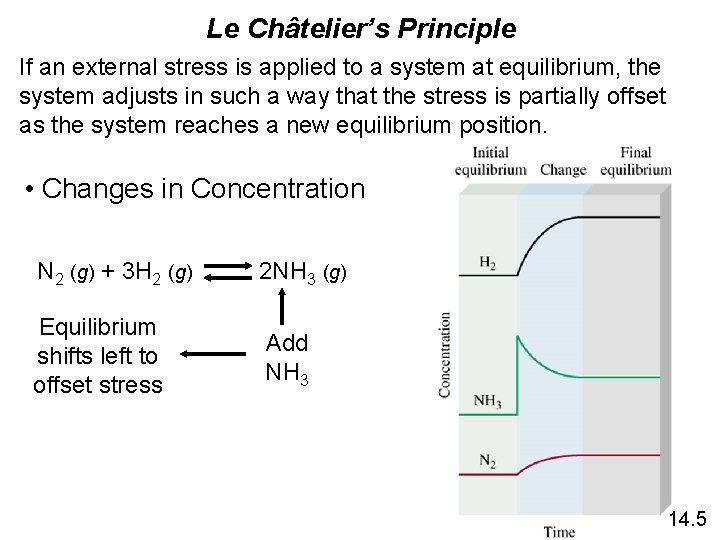
Le Châtelier’s Principle If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position. • Changes in Concentration N 2 (g) + 3 H 2 (g) 2 NH 3 (g) Equilibrium shifts left to offset stress Add NH 3 14. 5
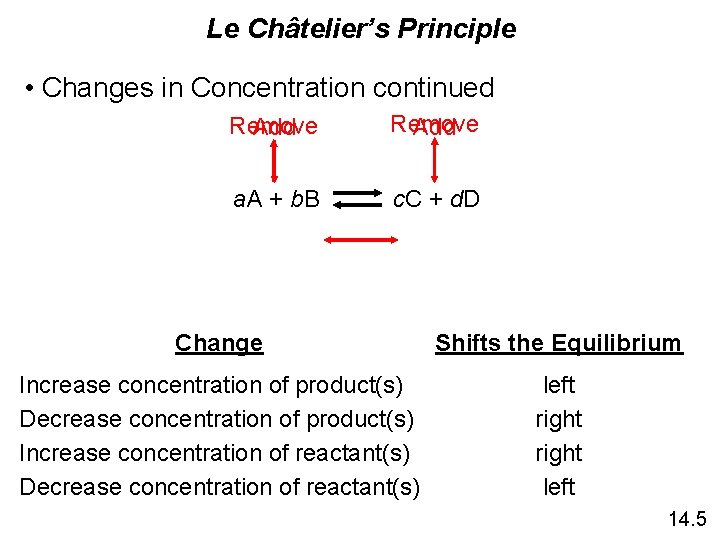
Le Châtelier’s Principle • Changes in Concentration continued Remove Add a. A + b. B c. C + d. D Change Shifts the Equilibrium Increase concentration of product(s) Decrease concentration of product(s) Increase concentration of reactant(s) Decrease concentration of reactant(s) left right left 14. 5
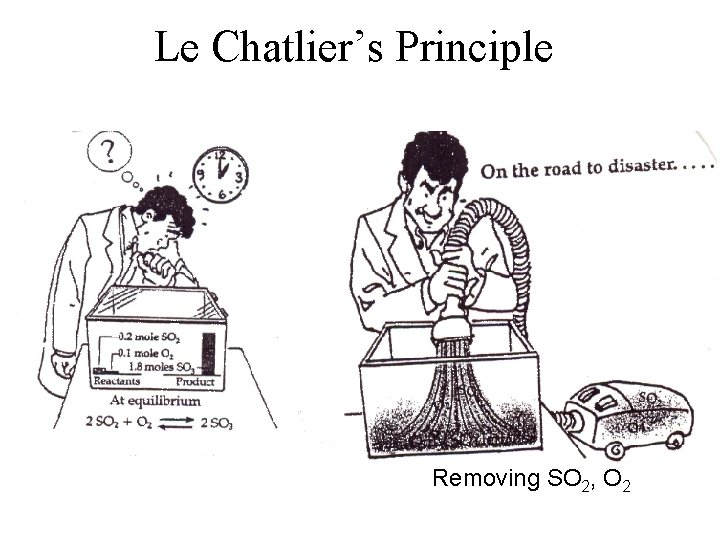
Le Chatlier’s Principle Removing SO 2, O 2

Le Chatlier’s Principle (cont)
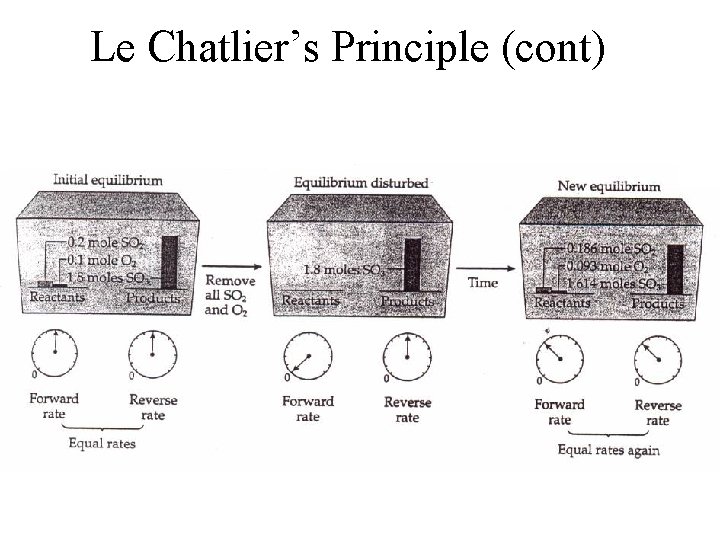
Le Chatlier’s Principle (cont)
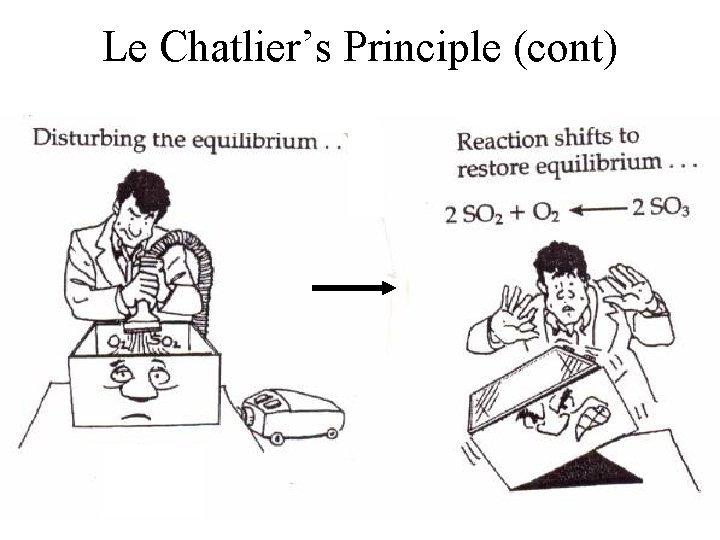
Le Chatlier’s Principle (cont)
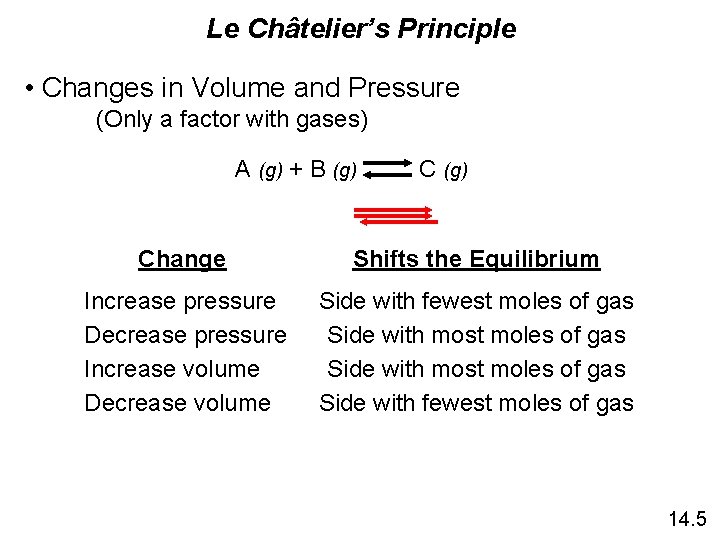
Le Châtelier’s Principle • Changes in Volume and Pressure (Only a factor with gases) A (g) + B (g) Change Shifts the Equilibrium Increase pressure Decrease pressure Increase volume Decrease volume Side with fewest moles of gas Side with most moles of gas Side with fewest moles of gas 14. 5
Le Châtelier’s Principle Chemistry; The Science in Context; by Thomas R. Gilbert, Rein V. Kirss, and Geoffrey Davies, Norton Publisher, 2004, p 752
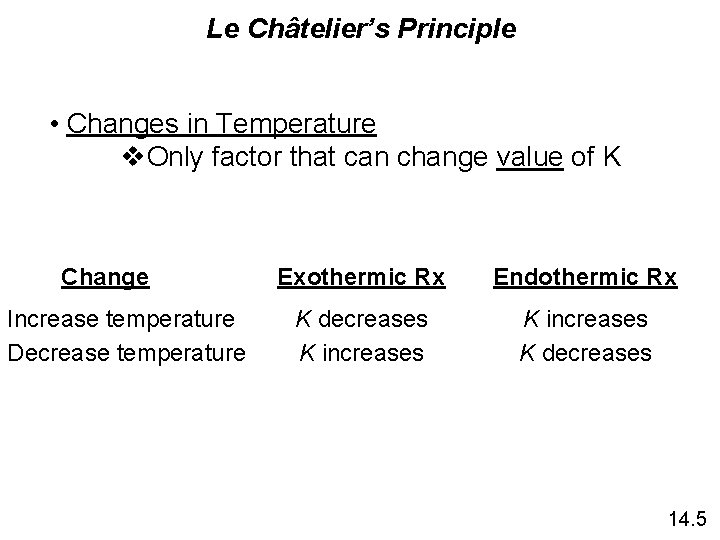
Le Châtelier’s Principle • Changes in Temperature v. Only factor that can change value of K Change Increase temperature Decrease temperature Exothermic Rx Endothermic Rx K decreases K increases K decreases 14. 5
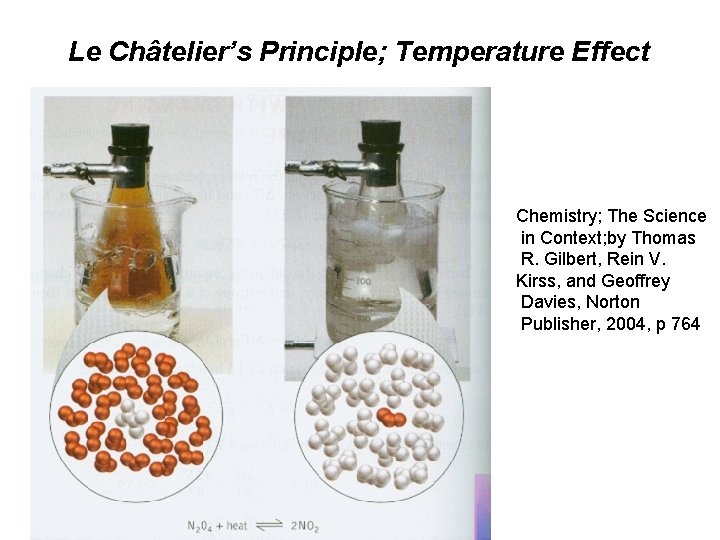
Le Châtelier’s Principle; Temperature Effect Chemistry; The Science in Context; by Thomas R. Gilbert, Rein V. Kirss, and Geoffrey Davies, Norton Publisher, 2004, p 764

Le Châtelier’s Principle • Adding a Catalyst • does not change K • does not shift the position of an equilibrium system • system will reach equilibrium sooner
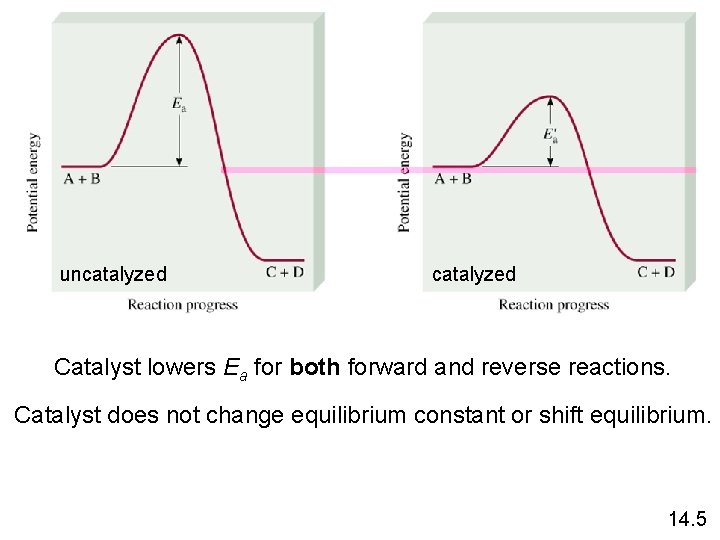
uncatalyzed Catalyst lowers Ea for both forward and reverse reactions. Catalyst does not change equilibrium constant or shift equilibrium. 14. 5
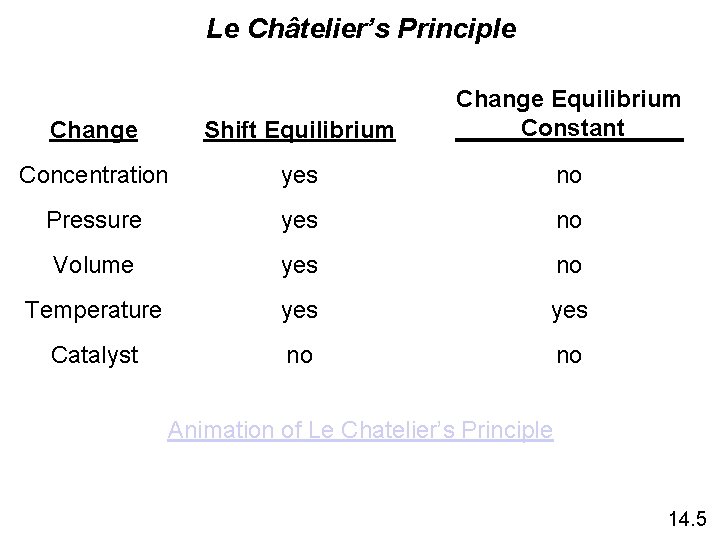
Le Châtelier’s Principle Change Shift Equilibrium Change Equilibrium Constant Concentration yes no Pressure yes no Volume yes no Temperature yes Catalyst no no Animation of Le Chatelier’s Principle 14. 5
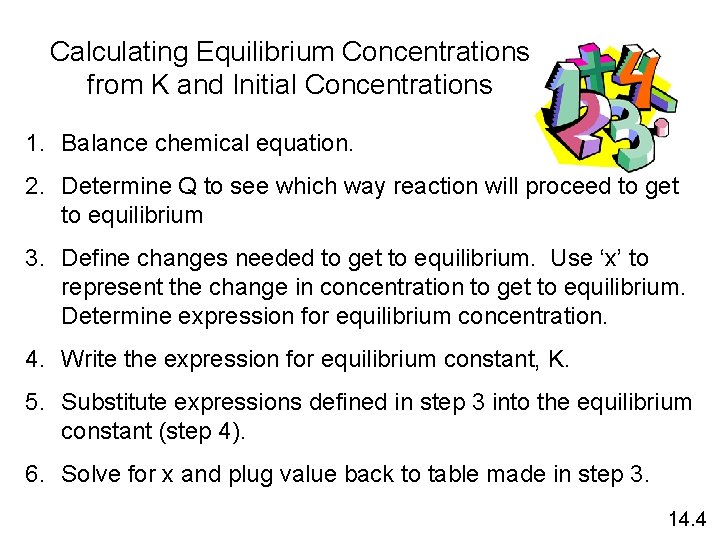
Calculating Equilibrium Concentrations from K and Initial Concentrations 1. Balance chemical equation. 2. Determine Q to see which way reaction will proceed to get to equilibrium 3. Define changes needed to get to equilibrium. Use ‘x’ to represent the change in concentration to get to equilibrium. Determine expression for equilibrium concentration. 4. Write the expression for equilibrium constant, K. 5. Substitute expressions defined in step 3 into the equilibrium constant (step 4). 6. Solve for x and plug value back to table made in step 3. 14. 4
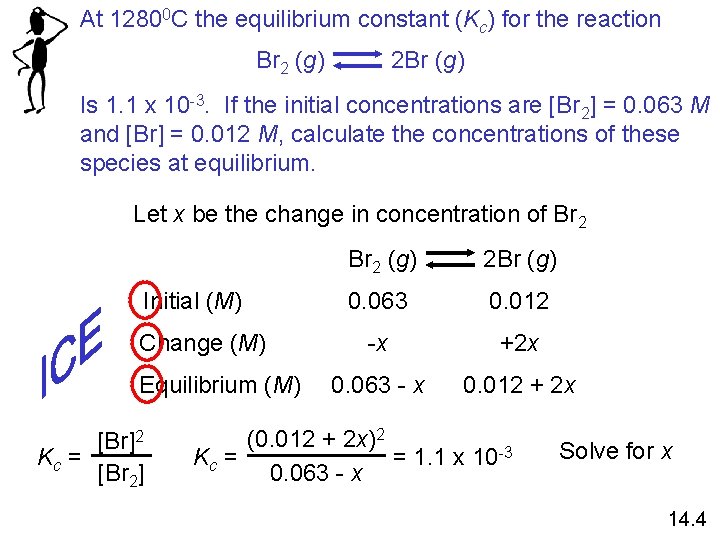
At 12800 C the equilibrium constant (Kc) for the reaction Br 2 (g) 2 Br (g) Is 1. 1 x 10 -3. If the initial concentrations are [Br 2] = 0. 063 M and [Br] = 0. 012 M, calculate the concentrations of these species at equilibrium. Let x be the change in concentration of Br 2 Initial (M) Change (M) Equilibrium (M) [Br]2 Kc = [Br 2] Br 2 (g) 2 Br (g) 0. 063 0. 012 -x +2 x 0. 063 - x 0. 012 + 2 x (0. 012 + 2 x)2 = 1. 1 x 10 -3 Kc = 0. 063 - x Solve for x 14. 4
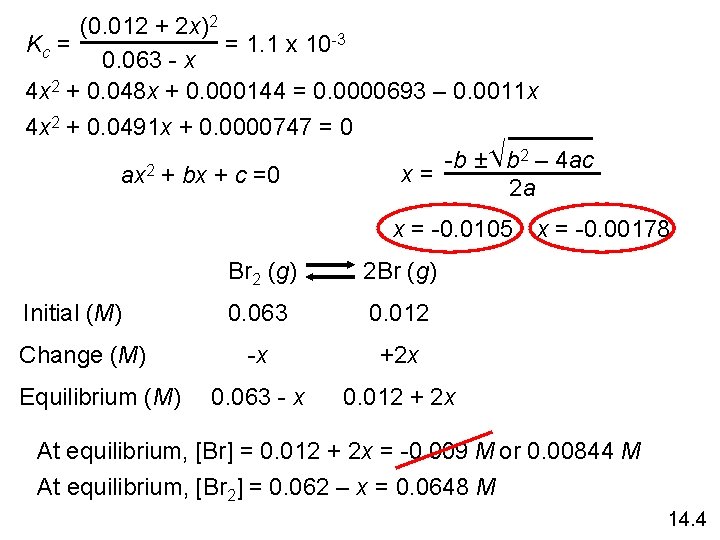
(0. 012 + 2 x)2 = 1. 1 x 10 -3 Kc = 0. 063 - x 4 x 2 + 0. 048 x + 0. 000144 = 0. 0000693 – 0. 0011 x 4 x 2 + 0. 0491 x + 0. 0000747 = 0 -b ± b 2 – 4 ac 2 x= ax + bx + c =0 2 a x = -0. 0105 x = -0. 00178 Initial (M) Change (M) Equilibrium (M) Br 2 (g) 2 Br (g) 0. 063 0. 012 -x +2 x 0. 063 - x 0. 012 + 2 x At equilibrium, [Br] = 0. 012 + 2 x = -0. 009 M or 0. 00844 M At equilibrium, [Br 2] = 0. 062 – x = 0. 0648 M 14. 4
- Slides: 56