Chemical Equilibrium Lesson 8 Acid Equilibrium Calculations Percent
Chemical Equilibrium Lesson # 8 Acid Equilibrium Calculations

Percent Ionization • When we calculate p. H of acids, we generally can only do so directly if it is a strong acid. • This is because strong acids ionize completely in water, meaning that the concentration of hydrogen ions in solution is equal to the given concentration of the acid. • With weak acids, we first need to know the percent ionization.
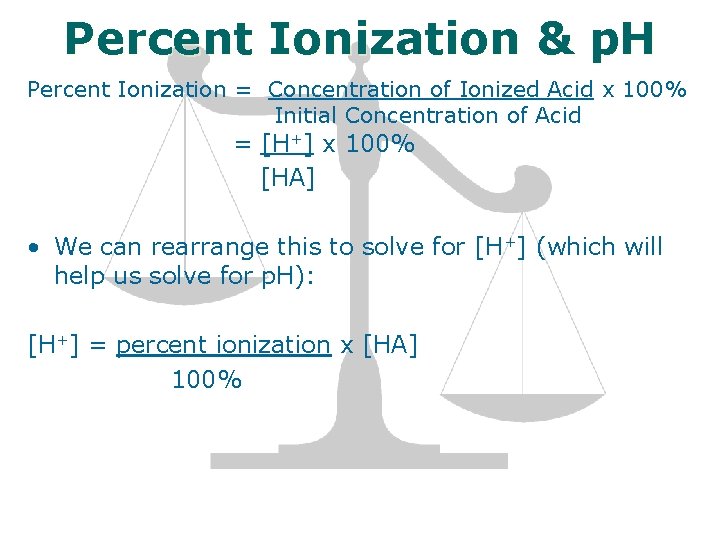
Percent Ionization & p. H Percent Ionization = Concentration of Ionized Acid x 100% Initial Concentration of Acid = [H+] x 100% [HA] • We can rearrange this to solve for [H+] (which will help us solve for p. H): [H+] = percent ionization x [HA] 100%

Example 1 A chemist prepares a 0. 10 mol/L solution of methanoic acid. The p. H of the solution is 2. 38. Determine the percentage ionization of the acid.

Example 2 A chemistry student prepares a solution of ethanoic acid, HC 2 H 3 O 2, to a concentration of 0. 1000 mol/L. If the percentage ionization of ethanoic acid is 1. 3%, what is the acid ionization constant, Ka, for ethanoic acid?

Example 3 The standard value for the Ka of HF is 6. 6 x 10 -4. Calculate the p. H of a 1. 00 mol/L solution of HF.

Example 4 A solution of hypochlorous acid, HCl. O (aq), has a concentration of 0. 100 mol/L. If the p. H of the solution is 4. 23, calculate the Ka of hypochlorous acid.
- Slides: 7