Chemical Equilibrium Hydrogen ions and acidity An acid

Chemical Equilibrium
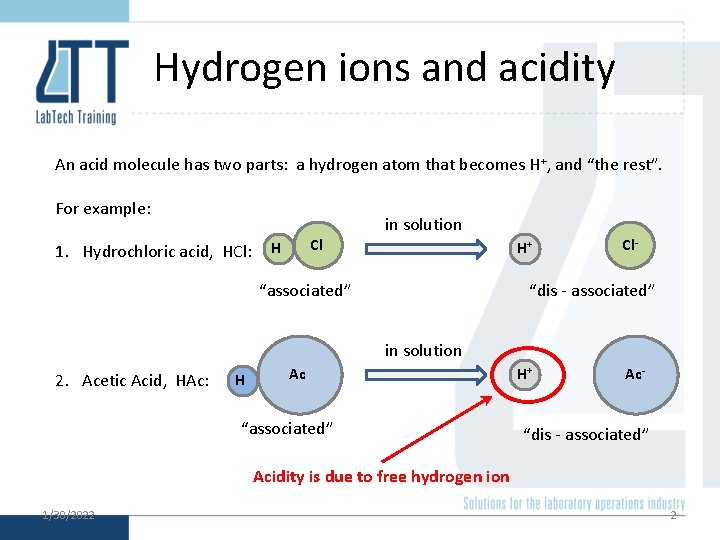
Hydrogen ions and acidity An acid molecule has two parts: a hydrogen atom that becomes H+, and “the rest”. For example: 1. Hydrochloric acid, HCl: Cl H in solution H+ “associated” Cl- “dis - associated” in solution 2. Acetic Acid, HAc: H Ac “associated” H+ Ac- “dis - associated” Acidity is due to free hydrogen ion 1/30/2022 2
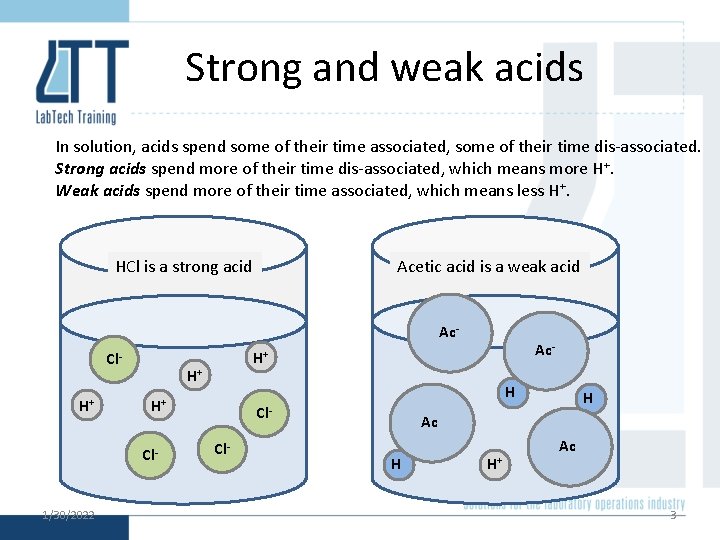
Strong and weak acids In solution, acids spend some of their time associated, some of their time dis-associated. Strong acids spend more of their time dis-associated, which means more H+. Weak acids spend more of their time associated, which means less H+. HCl is a strong acid Acetic acid is a weak acid Ac- Cl. H+ H+ 1/30/2022 H H+ Cl- Ac- H+ Cl. Cl- H Ac H H+ Ac 3
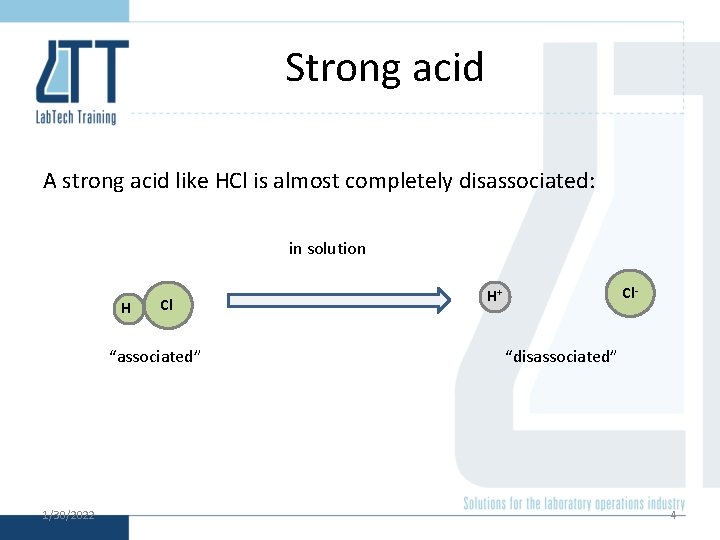
Strong acid A strong acid like HCl is almost completely disassociated: in solution H Cl “associated” 1/30/2022 Cl- H+ “disassociated” 4
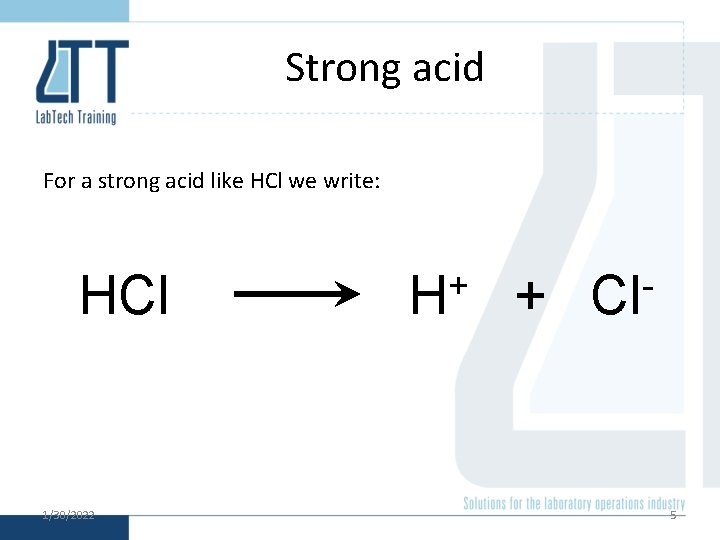
Strong acid For a strong acid like HCl we write: HCl 1/30/2022 + H + Cl 5
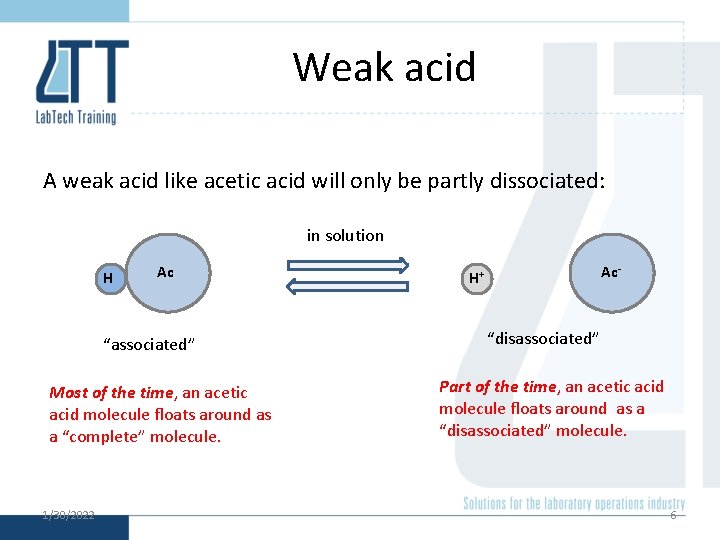
Weak acid A weak acid like acetic acid will only be partly dissociated: in solution H Ac “associated” Most of the time, an acetic acid molecule floats around as a “complete” molecule. 1/30/2022 Ac- H+ “disassociated” Part of the time, an acetic acid molecule floats around as a “disassociated” molecule. 6
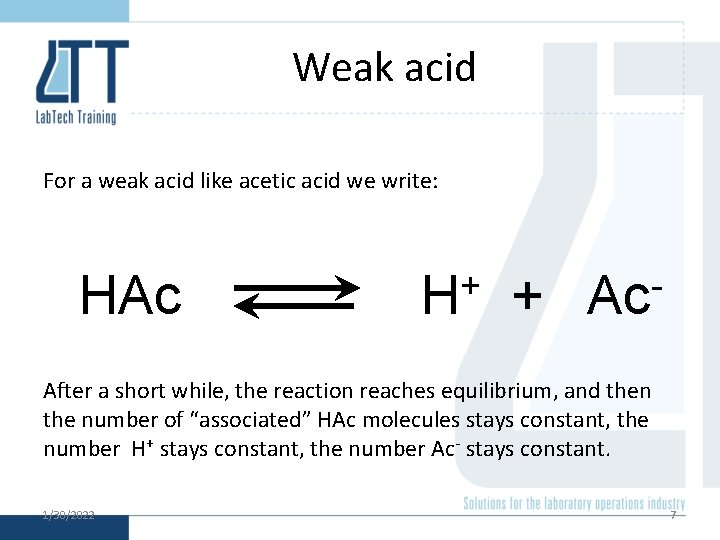
Weak acid For a weak acid like acetic acid we write: HAc + H + Ac After a short while, the reaction reaches equilibrium, and then the number of “associated” HAc molecules stays constant, the number H+ stays constant, the number Ac- stays constant. 1/30/2022 7
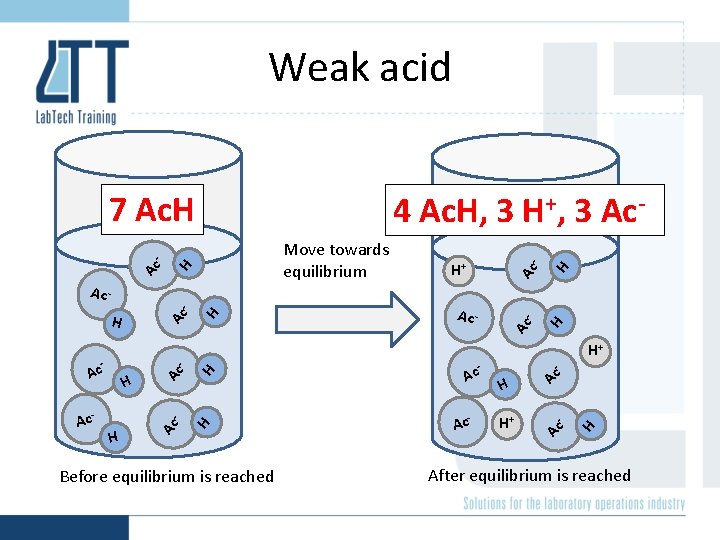
Weak acid Ac - H+ Ac - H Before equilibrium is reached - Ac H H H - Ac Ac - - H H Ac - - Ac - H+ Ac Ac Ac - H H H Ac - H+ Ac - Move towards equilibrium H 4 Ac. H, 3 H+, 3 Ac- H Ac - 7 Ac. H After equilibrium is reached
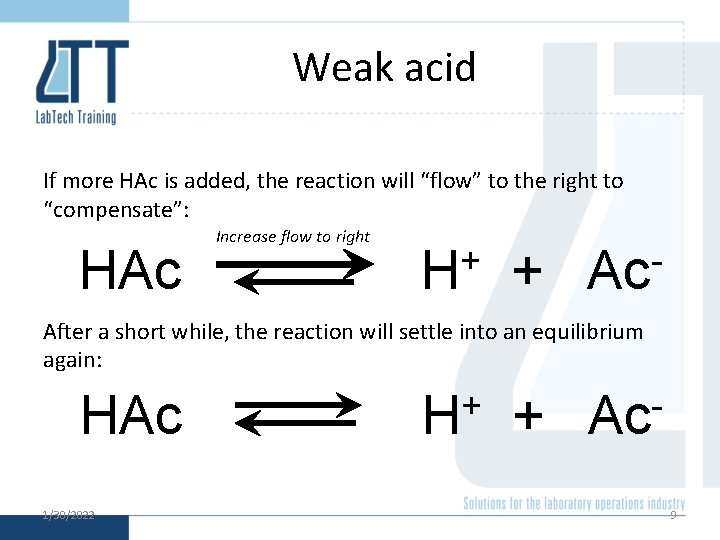
Weak acid If more HAc is added, the reaction will “flow” to the right to “compensate”: HAc Increase flow to right + H + Ac After a short while, the reaction will settle into an equilibrium again: HAc 1/30/2022 + H + Ac 9
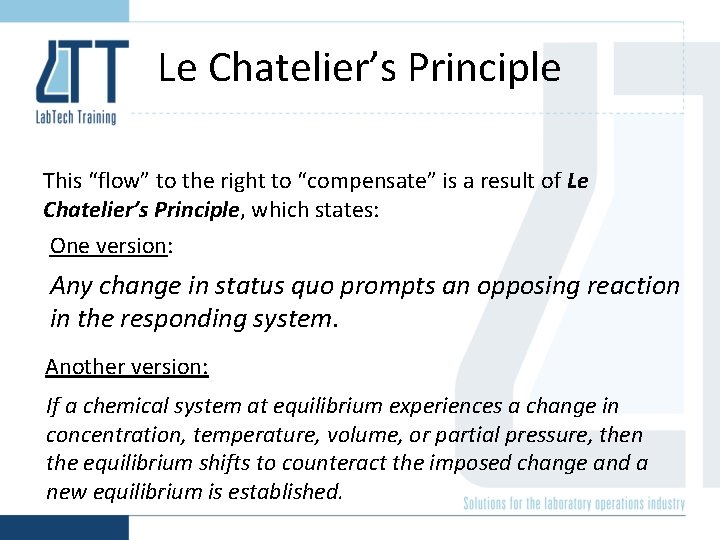
Le Chatelier’s Principle This “flow” to the right to “compensate” is a result of Le Chatelier’s Principle, which states: One version: Any change in status quo prompts an opposing reaction in the responding system. Another version: If a chemical system at equilibrium experiences a change in concentration, temperature, volume, or partial pressure, then the equilibrium shifts to counteract the imposed change and a new equilibrium is established.
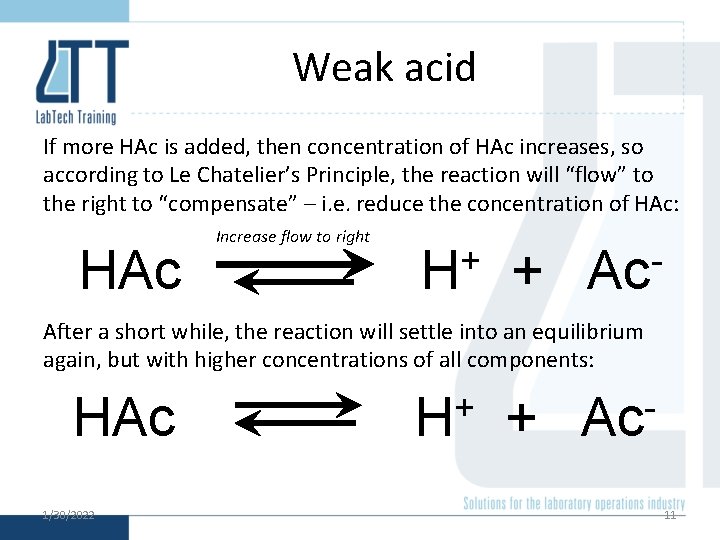
Weak acid If more HAc is added, then concentration of HAc increases, so according to Le Chatelier’s Principle, the reaction will “flow” to the right to “compensate” – i. e. reduce the concentration of HAc: HAc Increase flow to right + H + Ac After a short while, the reaction will settle into an equilibrium again, but with higher concentrations of all components: HAc 1/30/2022 + H + Ac 11
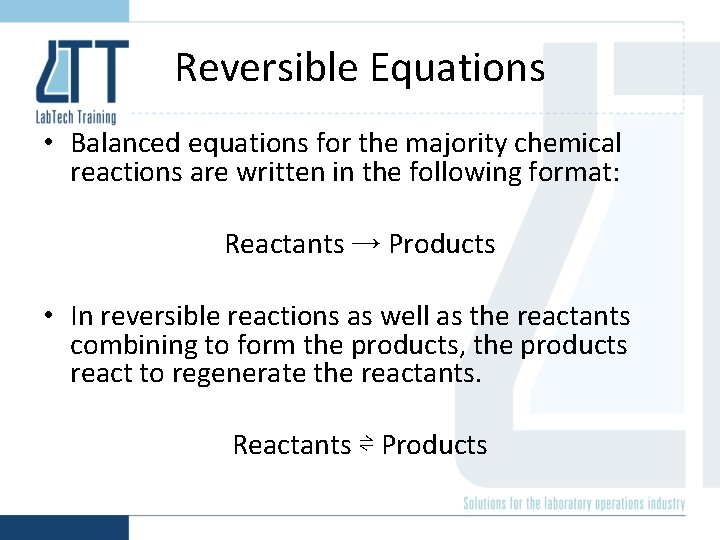
Reversible Equations • Balanced equations for the majority chemical reactions are written in the following format: Reactants → Products • In reversible reactions as well as the reactants combining to form the products, the products react to regenerate the reactants. Reactants ⇌ Products
Equilibrium • A system is said to be in equilibrium when at a given temperature, the concentration of reactants and products remain constant. – At this point the reactants are forming products at the same rate as products are being converted back to reactants.
Equilibrium • Equilibrium: – occurs in a closed system where no materials can get in or out. – is a dynamic state where forward and reverse reactions occur at the same time. – mixtures can comprise solids, liquids, gases or aqueous solutions.
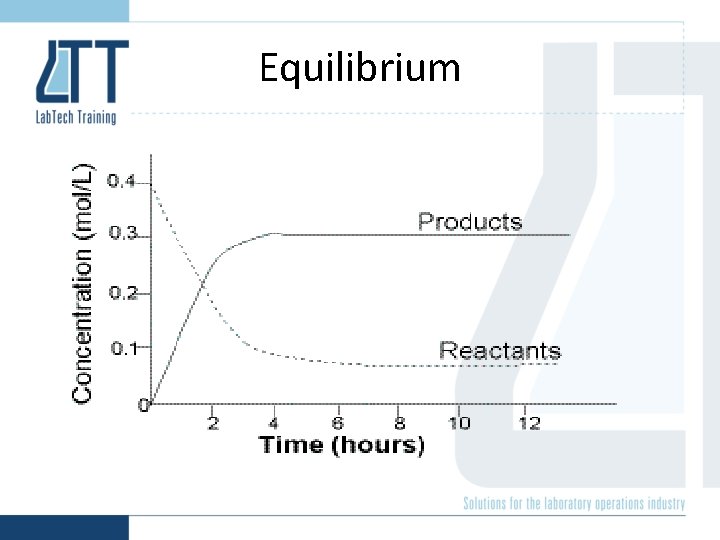
Equilibrium
![Equilibrium Constant We can write the concentration of HAc as [HAc]. We can write Equilibrium Constant We can write the concentration of HAc as [HAc]. We can write](http://slidetodoc.com/presentation_image_h2/963a02203431cd2304866ff95e7e05bf/image-16.jpg)
Equilibrium Constant We can write the concentration of HAc as [HAc]. We can write the concentration of H+ as [H+]. We can write the concentration of Ac- as [Ac-]. It turns out that the following is a constant at a particular temperature, @ equilibrium: Equilibrium Constant This equation “captures” Le Chatelier’s Principle. 1/30/2022 16
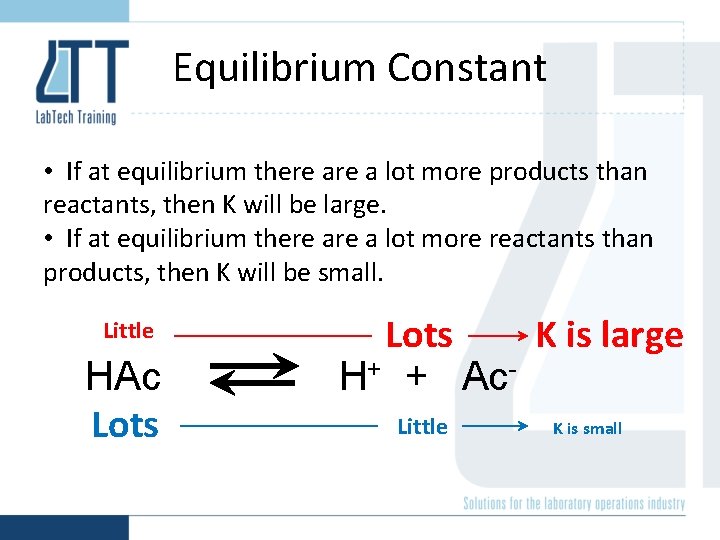
Equilibrium Constant • If at equilibrium there a lot more products than reactants, then K will be large. • If at equilibrium there a lot more reactants than products, then K will be small. Little HAc Lots K is large H+ + Ac. Little K is small
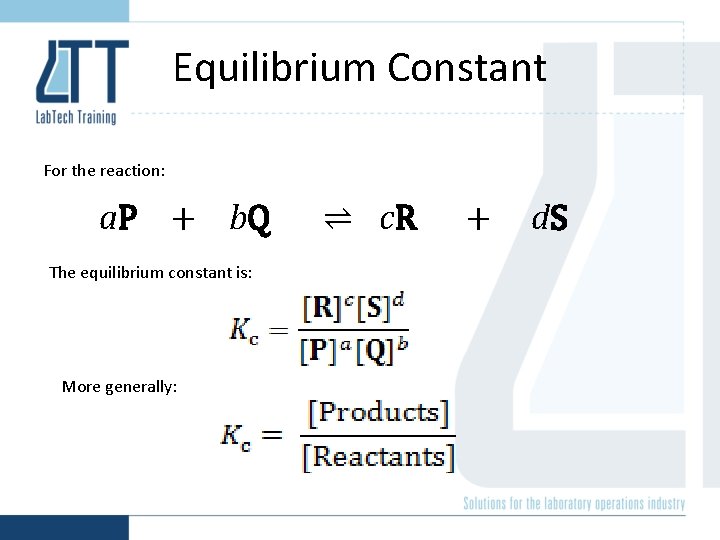
Equilibrium Constant For the reaction: a. P + b. Q The equilibrium constant is: More generally: ⇌ c. R + d. S
Question • Using the following equation, calculate the equilibrium constant. N 2(g) + 3 H 2(g) ⇌ 2 NH 3(g) • A one-liter vessel contains 1. 60 moles NH 3, 0. 80 moles N 2, and 1. 20 moles of H 2. What is the equilibrium constant?
- Slides: 19