Chemical Equilibrium Equilibrium two opposing processes occurring at

Chemical Equilibrium

Equilibrium: • two opposing processes occurring at the same rate to achieve balance Players enter and leave the game, but… the same total number of players on field.
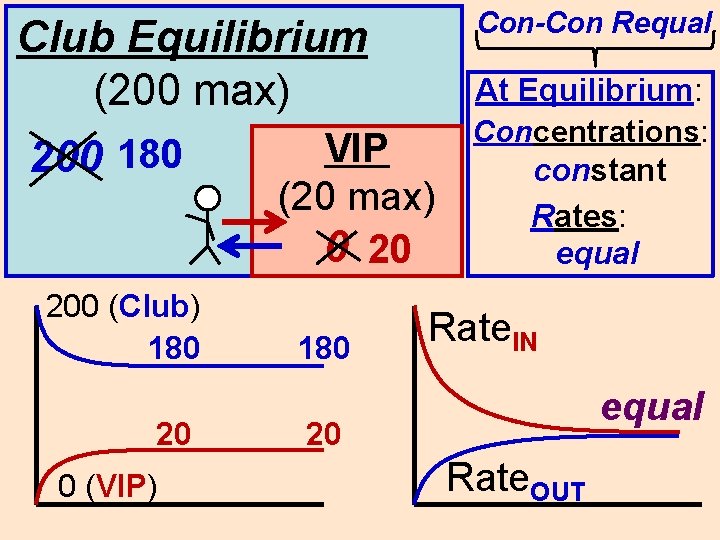
Con-Con Requal Club Equilibrium (200 max) 200 180 200 (Club) 180 20 0 (VIP) VIP (20 max) 0 20 180 At Equilibrium: Concentrations: constant Rates: equal Rate. IN equal 20 Rate. OUT
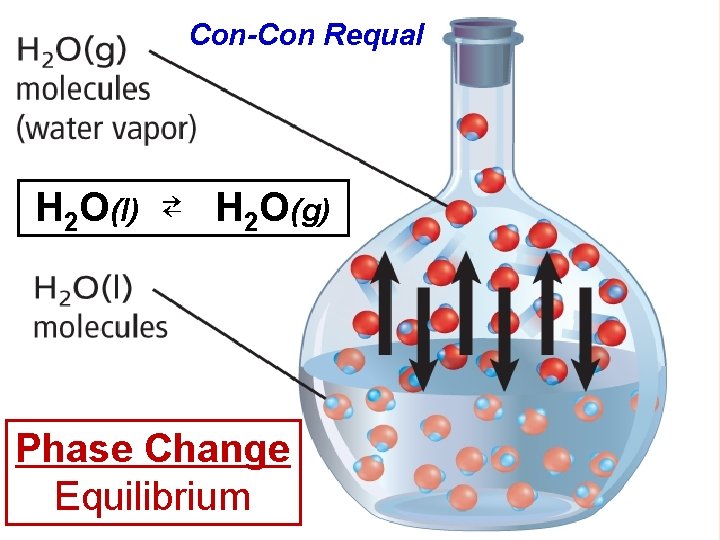
Con-Con Requal H 2 O(l) ⇄ H 2 O(g) Phase Change Equilibrium

Rate of Solvation = Rate of Crystallization Con-Con Requal • total moles of dissolved Na. Cl remains constant • total moles of undissolved Na. Cl(solid) remains constant Saturated Solution Equilibrium
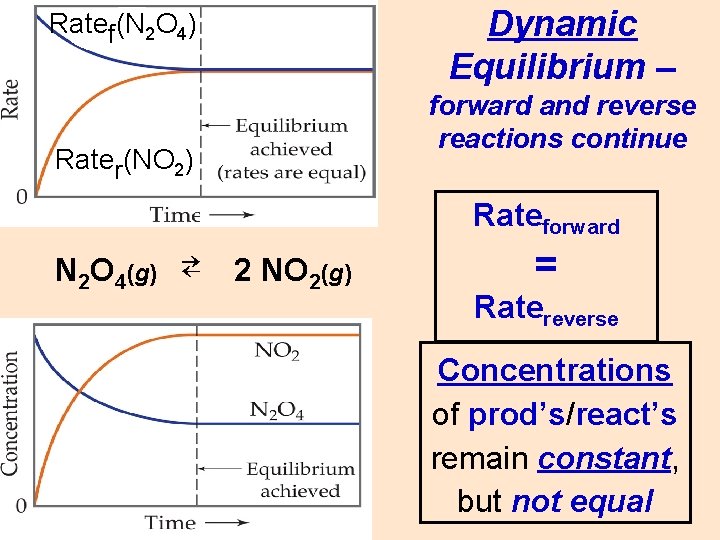
Dynamic Equilibrium – Ratef(N 2 O 4) forward and reverse reactions continue Rater(NO 2) Rateforward N 2 O 4(g) ⇄ 2 NO 2(g) = Ratereverse Concentrations of prod’s/react’s remain constant, but not equal

Quick Quiz! 1) When a reaction has reached equilibrium, reactants and products … A) have stopped reacting. B) continue forming. C) have equal concentrations. D) decrease in concentration.
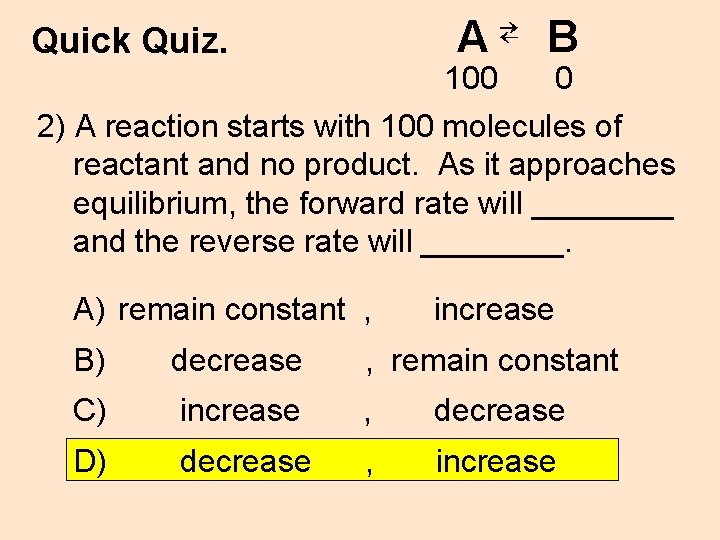
A⇄ B Quick Quiz. 100 0 2) A reaction starts with 100 molecules of reactant and no product. As it approaches equilibrium, the forward rate will ____ and the reverse rate will ____. A) remain constant , increase B) decrease , remain constant C) increase , decrease D) decrease , increase

Quick Quiz. A⇄ B 100 0 3) A reaction starts with 100 molecules of reactant and no product. At equilibrium, the amount of product B will be… A) greater than A B) less than A C) equal to A D) constant
- Slides: 9