Chemical Equilibrium Equilibrium Chemical of the forward and

+ Chemical Equilibrium
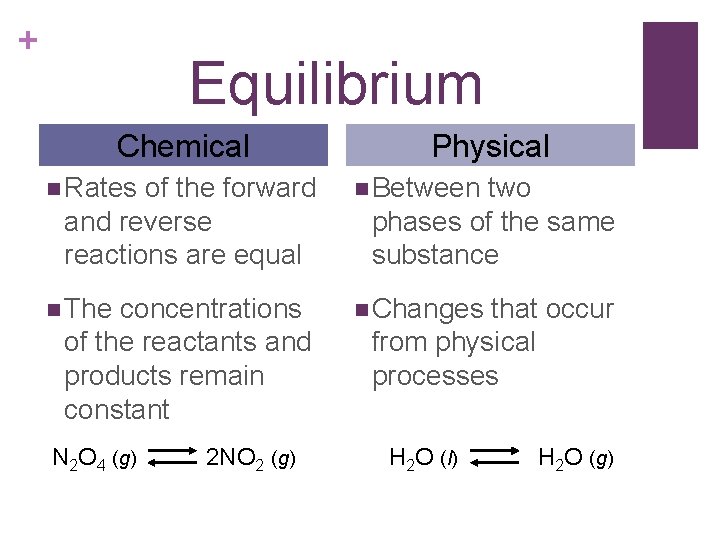
+ Equilibrium Chemical of the forward and reverse reactions are equal Physical n Rates n Between n The n Changes concentrations of the reactants and products remain constant N 2 O 4 (g) 2 NO 2 (g) two phases of the same substance that occur from physical processes H 2 O (l) H 2 O (g)
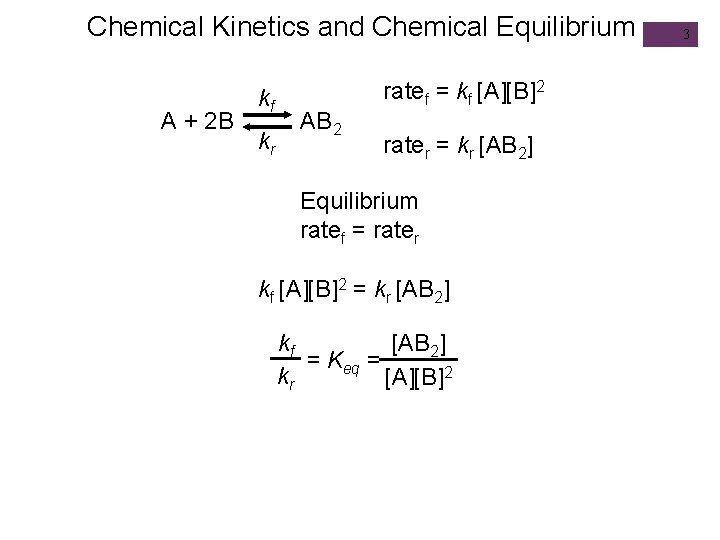
Chemical Kinetics and Chemical Equilibrium A + 2 B kf kr AB 2 ratef = kf [A][B]2 rater = kr [AB 2] Equilibrium ratef = rater kf [A][B]2 = kr [AB 2] kf [AB 2] = Keq = kr [A][B]2 3

N 2 O 4 (g) 2 NO 2 (g) 4 equilibrium Start with N 2 O 4 Start with NO 2 & N 2 O 4 equilibrium Start with NO 2
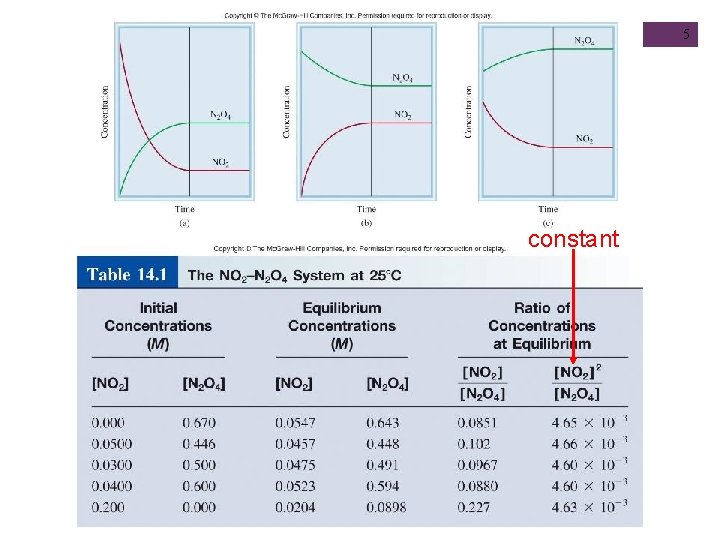
5 constant
![N 2 O 4 (g) Keq = [NO 2]2 [N 2 O 4] a. N 2 O 4 (g) Keq = [NO 2]2 [N 2 O 4] a.](http://slidetodoc.com/presentation_image_h2/6d1de1bf9e2445b3d68f637df2118a86/image-6.jpg)
N 2 O 4 (g) Keq = [NO 2]2 [N 2 O 4] a. A + b. B Keq = 2 NO 2 (g) = 4. 63 x 10 -3 c. C + d. D [C]c[D]d [A]a[B]b 6
![7 Keq = [C]c[D]d a. A + b. B [A]a[B]b c. C + d. 7 Keq = [C]c[D]d a. A + b. B [A]a[B]b c. C + d.](http://slidetodoc.com/presentation_image_h2/6d1de1bf9e2445b3d68f637df2118a86/image-7.jpg)
7 Keq = [C]c[D]d a. A + b. B [A]a[B]b c. C + d. D Equilibrium Will Keq >> 1 Lie to the right Favor products Keq << 1 Favor reactants Lie to the left
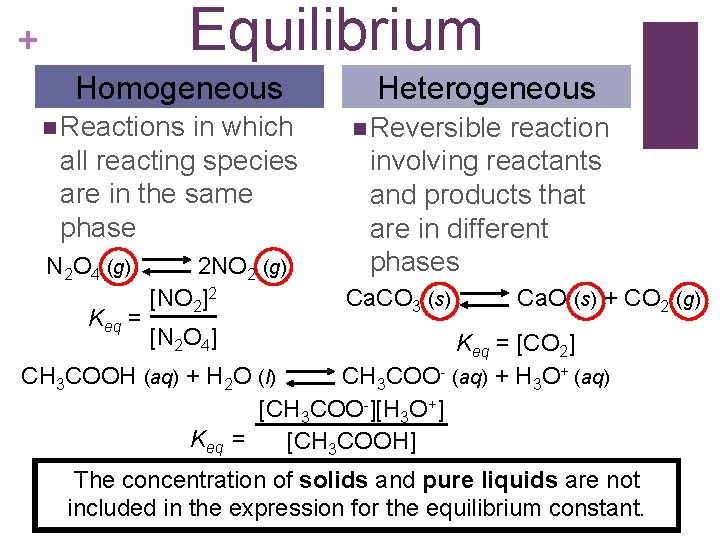
Equilibrium + Homogeneous n Reactions in which all reacting species are in the same phase N 2 O 4 (g) Keq = 2 NO 2 (g) [NO 2]2 Heterogeneous n Reversible reaction involving reactants and products that are in different phases Ca. CO 3 (s) Ca. O (s) + CO 2 (g) [N 2 O 4] Keq = [CO 2] CH 3 COOH (aq) + H 2 O (l) CH 3 COO- (aq) + H 3 O+ (aq) [CH 3 COO-][H 3 O+] Keq = [CH 3 COOH] The concentration of solids and pure liquids are not included in the expression for the equilibrium constant.
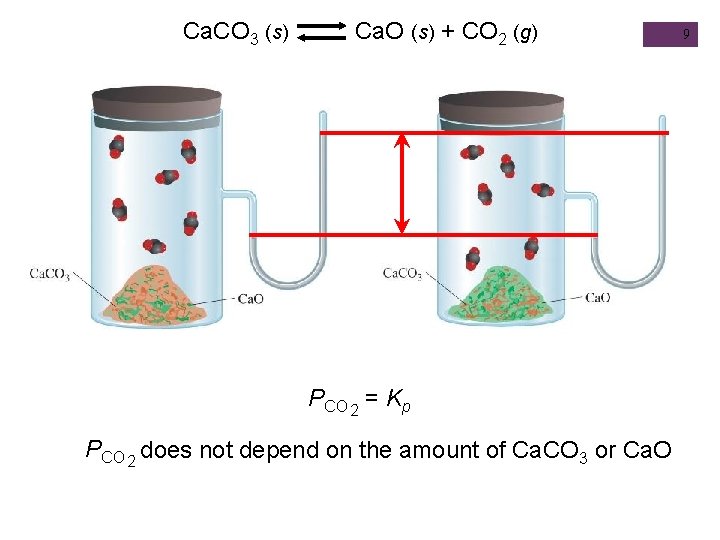
Ca. CO 3 (s) Ca. O (s) + CO 2 (g) PCO 2 = Kp PCO 2 does not depend on the amount of Ca. CO 3 or Ca. O 9
- Slides: 9