Chemical Equilibrium Chemical reactions often seem to stop
Chemical Equilibrium Chemical reactions often seem to stop before they are complete. Actually, such reactions are reversible. That is, the original reactants form products, but then the products react with themselves to give back the original reactants. When these two reactions—forward and reverse—occur at the same rate, a chemical equilibrium exists. שווי משקל כימי 11 - 1
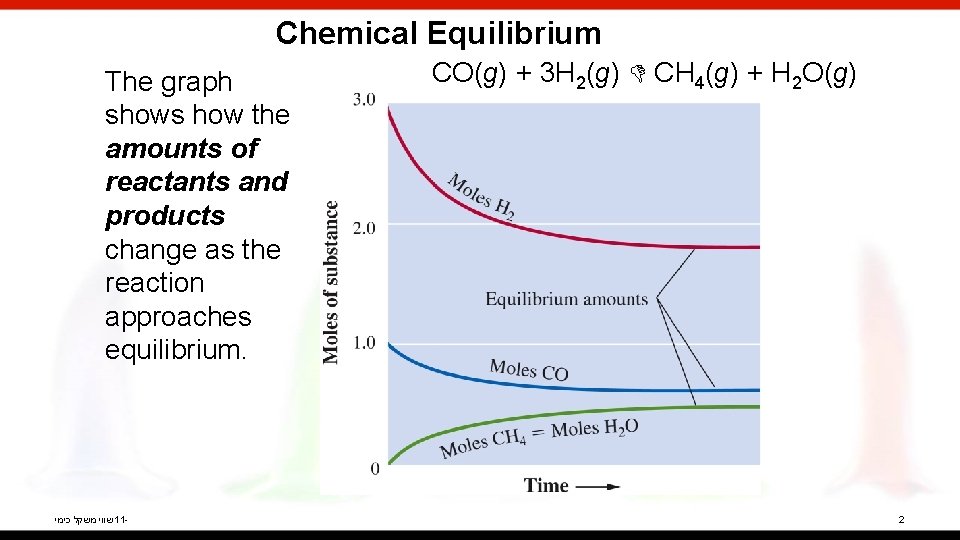
Chemical Equilibrium The graph shows how the amounts of reactants and products change as the reaction approaches equilibrium. שווי משקל כימי 11 - CO(g) + 3 H 2(g) CH 4(g) + H 2 O(g) 2
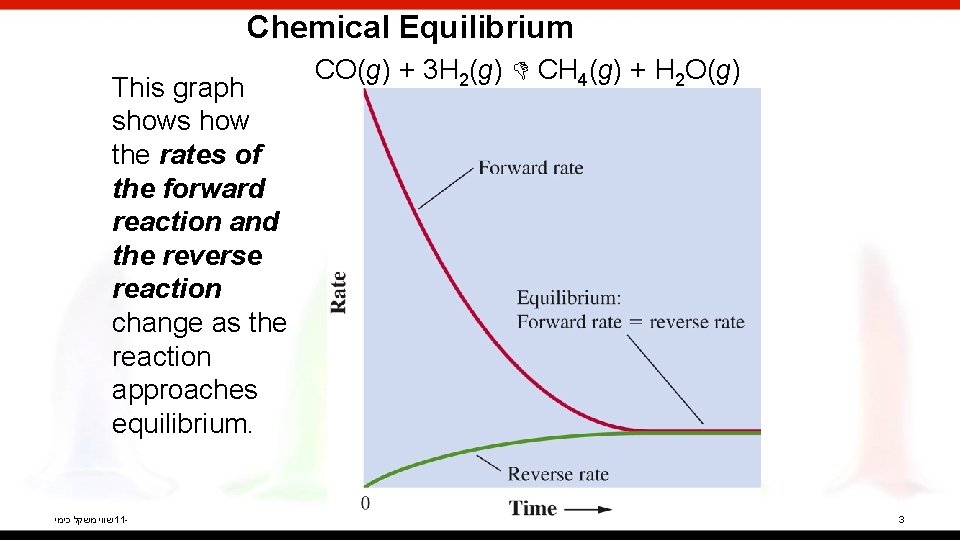
Chemical Equilibrium This graph shows how the rates of the forward reaction and the reverse reaction change as the reaction approaches equilibrium. שווי משקל כימי 11 - CO(g) + 3 H 2(g) CH 4(g) + H 2 O(g) 3

Chemical equilibrium is the state reached by a reaction mixture when the rates of the forward and reverse reactions have become equal. Continuous forward and reverse reactions make the equilibrium a dynamic process. שווי משקל כימי 11 - 4
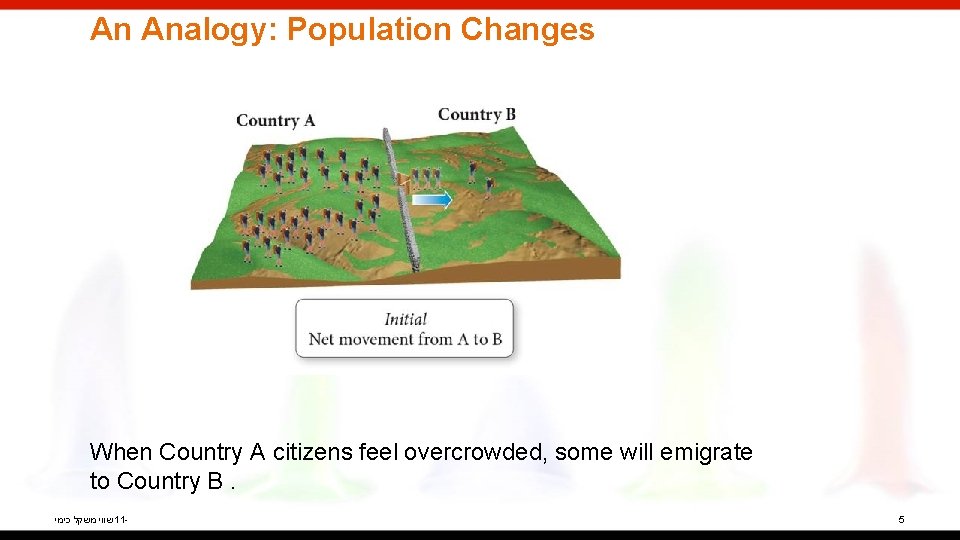
An Analogy: Population Changes When Country A citizens feel overcrowded, some will emigrate to Country B. שווי משקל כימי 11 - 5
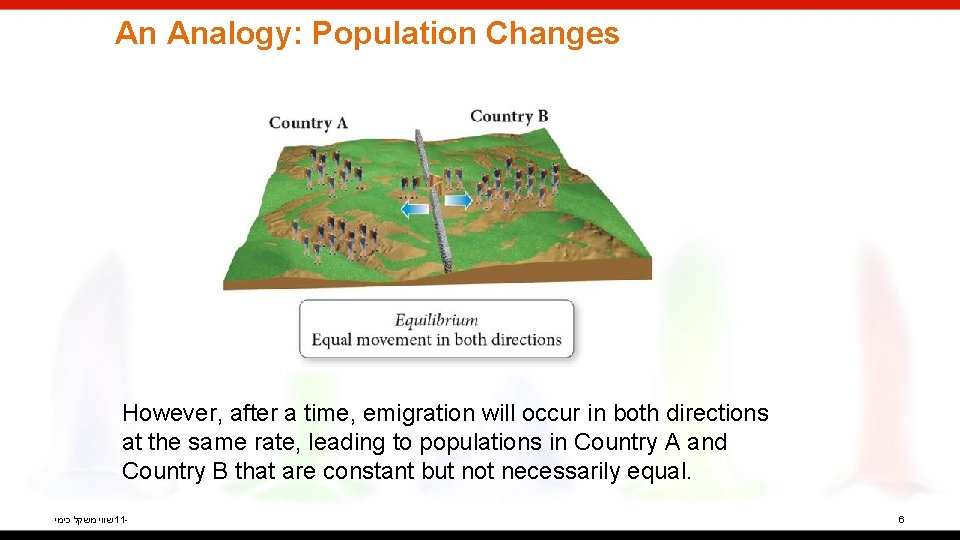
An Analogy: Population Changes However, after a time, emigration will occur in both directions at the same rate, leading to populations in Country A and Country B that are constant but not necessarily equal. שווי משקל כימי 11 - 6
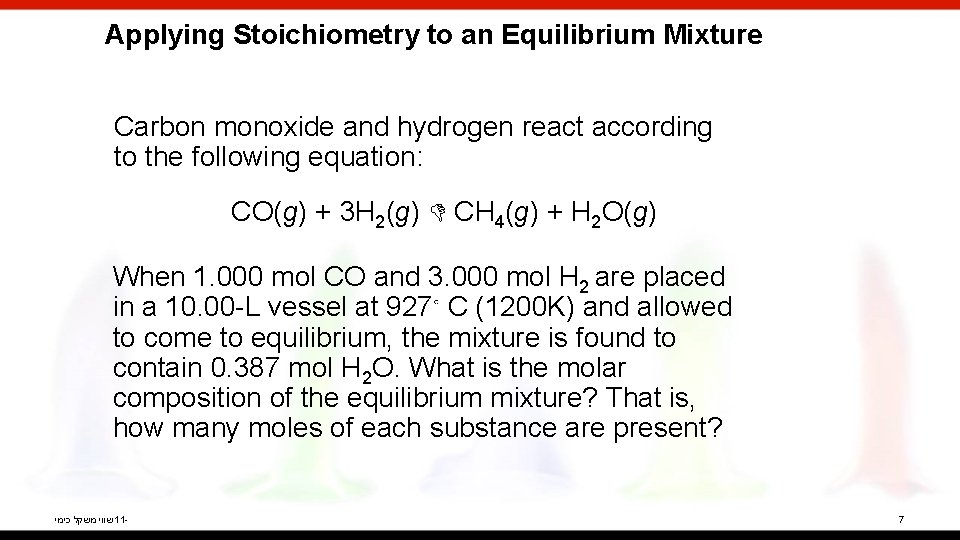
Applying Stoichiometry to an Equilibrium Mixture Carbon monoxide and hydrogen react according to the following equation: CO(g) + 3 H 2(g) CH 4(g) + H 2 O(g) When 1. 000 mol CO and 3. 000 mol H 2 are placed in a 10. 00 -L vessel at 927◦ C (1200 K) and allowed to come to equilibrium, the mixture is found to contain 0. 387 mol H 2 O. What is the molar composition of the equilibrium mixture? That is, how many moles of each substance are present? שווי משקל כימי 11 - 7
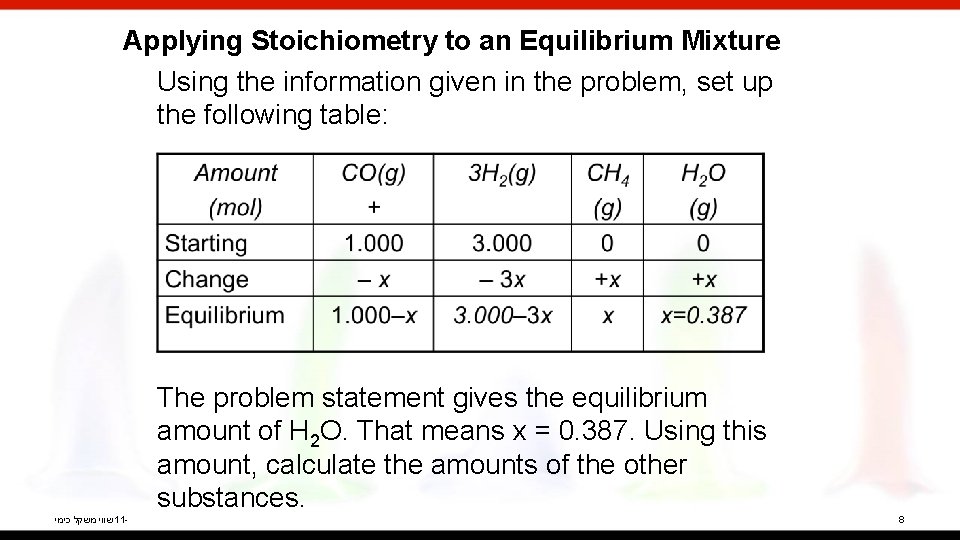
Applying Stoichiometry to an Equilibrium Mixture Using the information given in the problem, set up the following table: The problem statement gives the equilibrium amount of H 2 O. That means x = 0. 387. Using this amount, calculate the amounts of the other substances. שווי משקל כימי 11 - 8
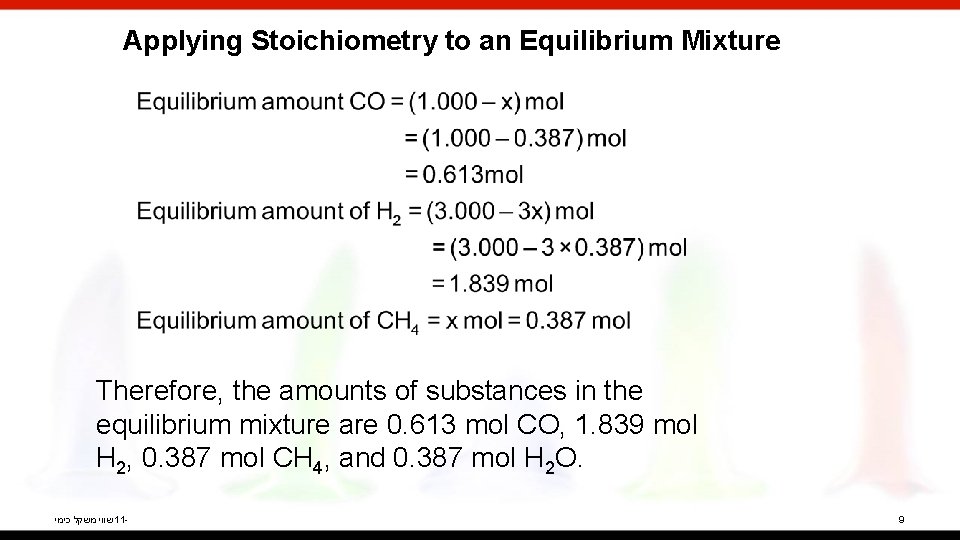
Applying Stoichiometry to an Equilibrium Mixture Therefore, the amounts of substances in the equilibrium mixture are 0. 613 mol CO, 1. 839 mol H 2, 0. 387 mol CH 4, and 0. 387 mol H 2 O. שווי משקל כימי 11 - 9
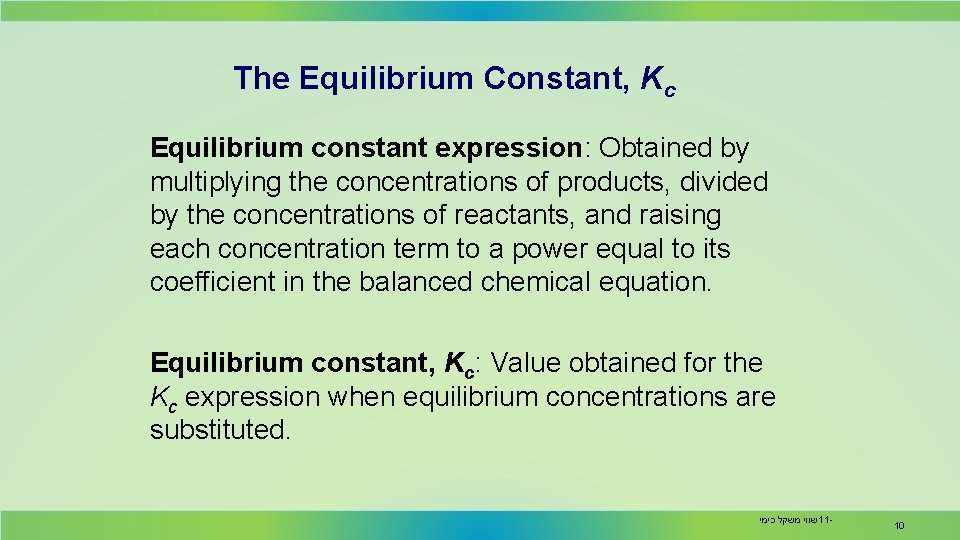
The Equilibrium Constant, Kc Equilibrium constant expression: Obtained by multiplying the concentrations of products, divided by the concentrations of reactants, and raising each concentration term to a power equal to its coefficient in the balanced chemical equation. Equilibrium constant, Kc: Value obtained for the Kc expression when equilibrium concentrations are substituted. שווי משקל כימי 11 - 10
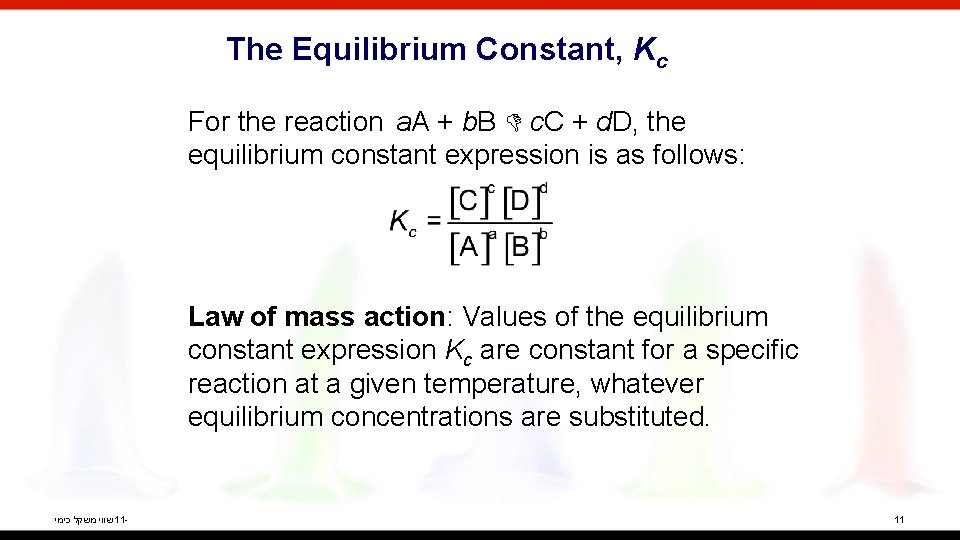
The Equilibrium Constant, Kc For the reaction a. A + b. B c. C + d. D, the equilibrium constant expression is as follows: Law of mass action: Values of the equilibrium constant expression Kc are constant for a specific reaction at a given temperature, whatever equilibrium concentrations are substituted. שווי משקל כימי 11 - 11

The Equilibrium Constant, Kc Write the equilibrium-constant expression Kc for catalytic methanation. CO(g) + 3 H 2(g) CH 4(g) + H 2 O(g) Solution The expression for the equilibrium constant is: שווי משקל כימי 11 - 12
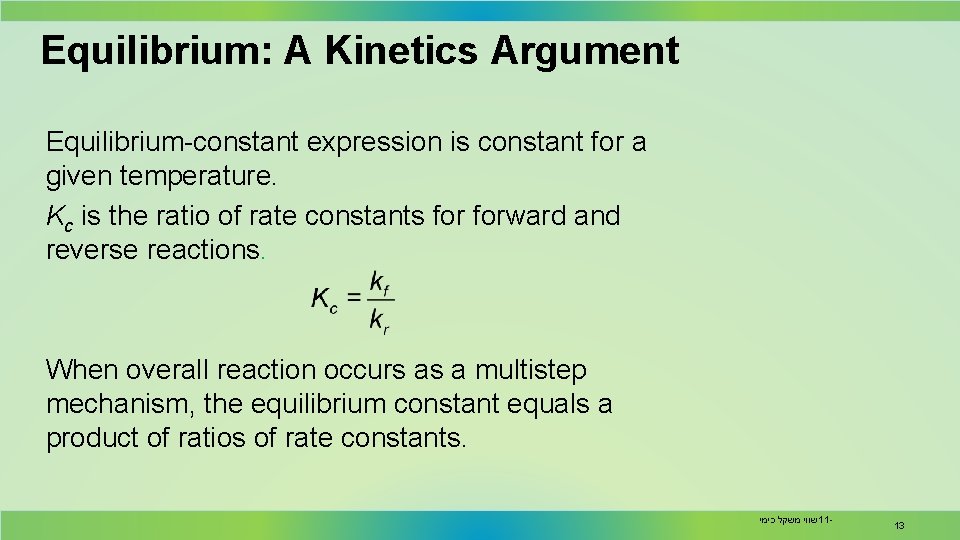
Equilibrium: A Kinetics Argument Equilibrium-constant expression is constant for a given temperature. Kc is the ratio of rate constants forward and reverse reactions. When overall reaction occurs as a multistep mechanism, the equilibrium constant equals a product of ratios of rate constants. שווי משקל כימי 11 - 13
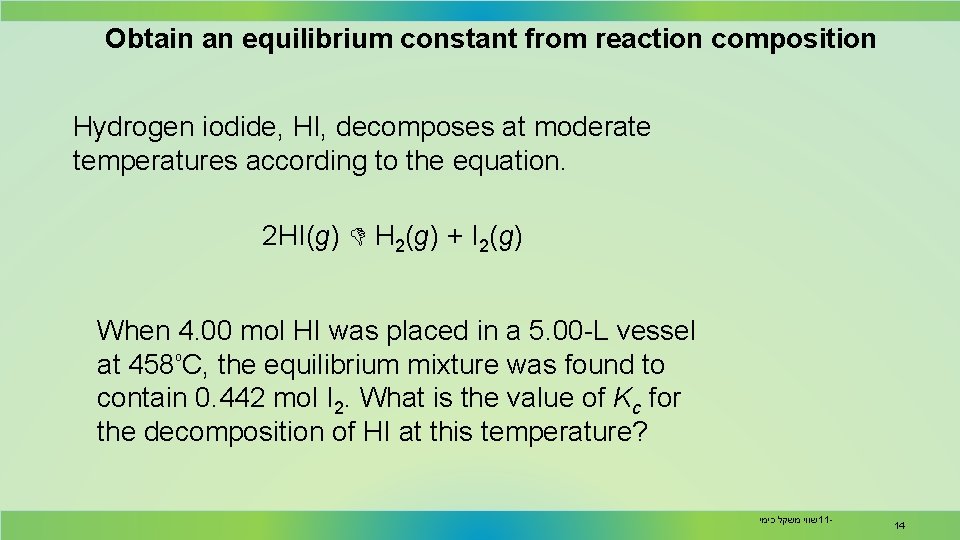
Obtain an equilibrium constant from reaction composition Hydrogen iodide, HI, decomposes at moderate temperatures according to the equation. 2 HI(g) H 2(g) + I 2(g) When 4. 00 mol HI was placed in a 5. 00 -L vessel ₒ at 458 C, the equilibrium mixture was found to contain 0. 442 mol I 2. What is the value of Kc for the decomposition of HI at this temperature? שווי משקל כימי 11 - 14
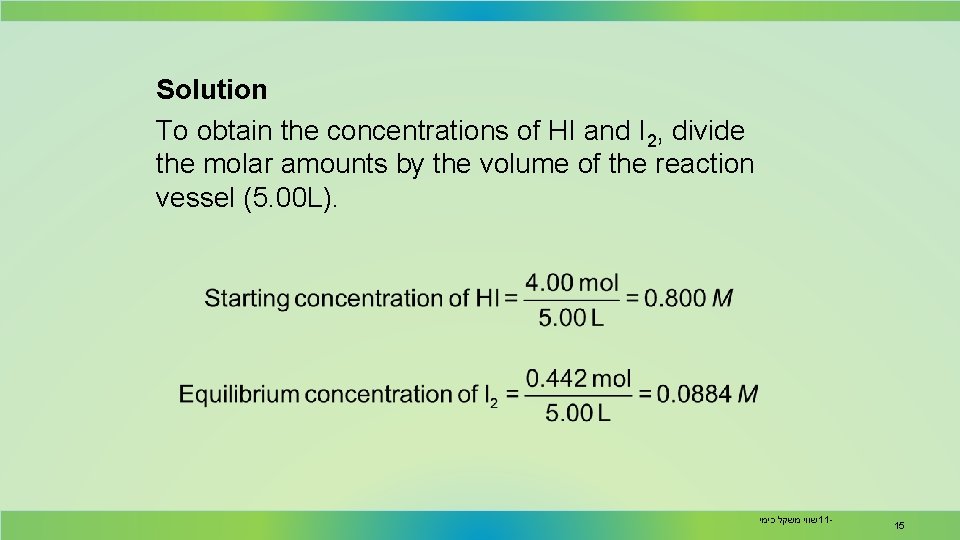
Solution To obtain the concentrations of HI and I 2, divide the molar amounts by the volume of the reaction vessel (5. 00 L). שווי משקל כימי 11 - 15
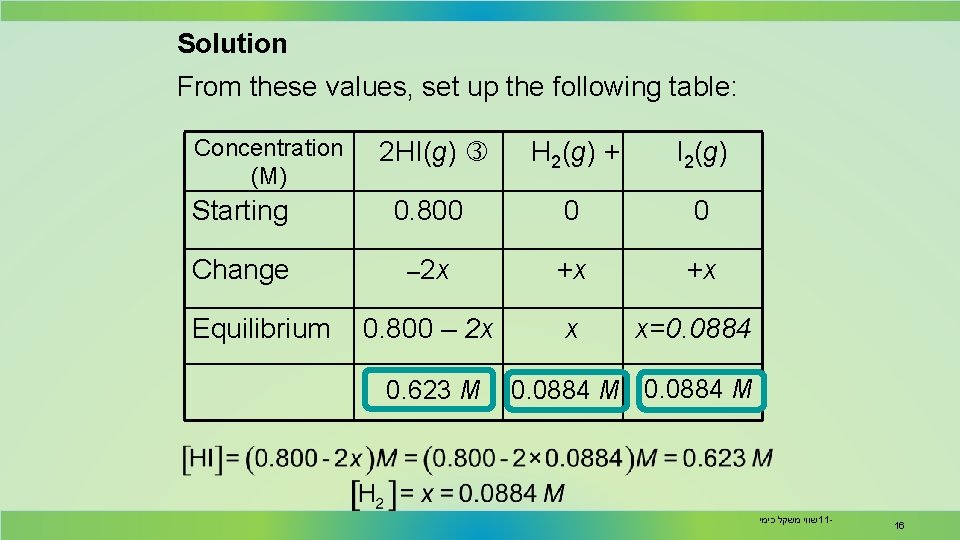
Solution From these values, set up the following table: Concentration (M) 2 HI(g) H 2(g) + I 2(g) Starting 0. 800 0 0 Change – 2 x +x +x 0. 800 – 2 x x Equilibrium 0. 623 M x=0. 0884 M שווי משקל כימי 11 - 16
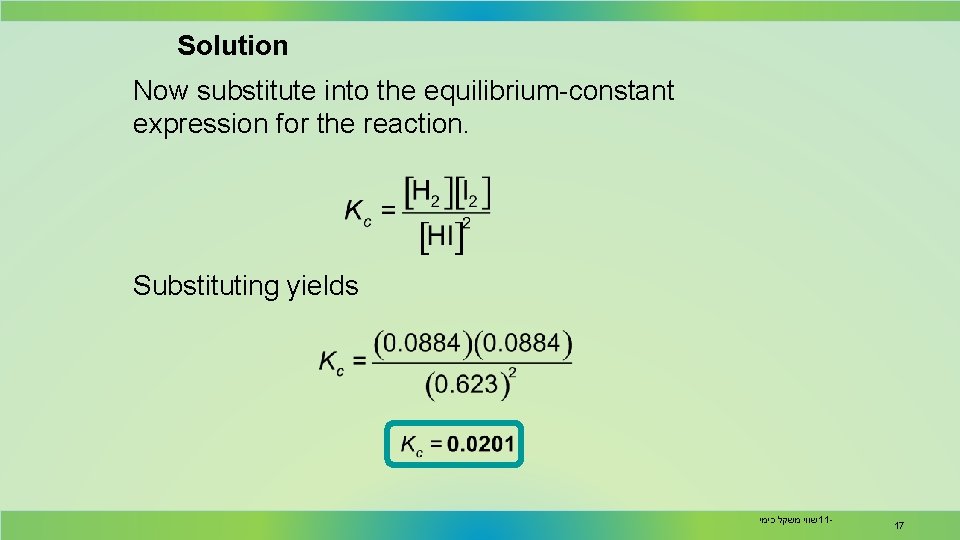
Solution Now substitute into the equilibrium-constant expression for the reaction. Substituting yields שווי משקל כימי 11 - 17
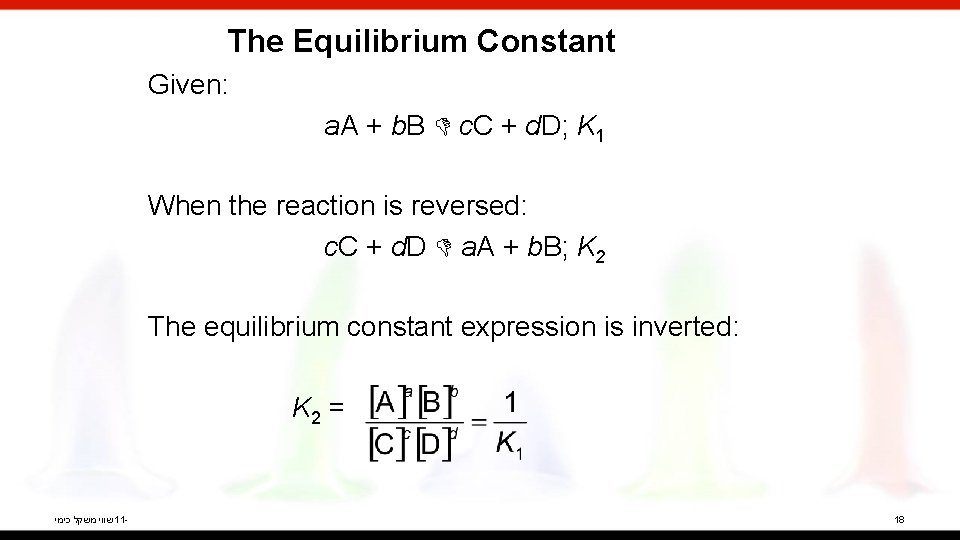
The Equilibrium Constant Given: a. A + b. B c. C + d. D; K 1 When the reaction is reversed: c. C + d. D a. A + b. B; K 2 The equilibrium constant expression is inverted: K 2 = שווי משקל כימי 11 - 18
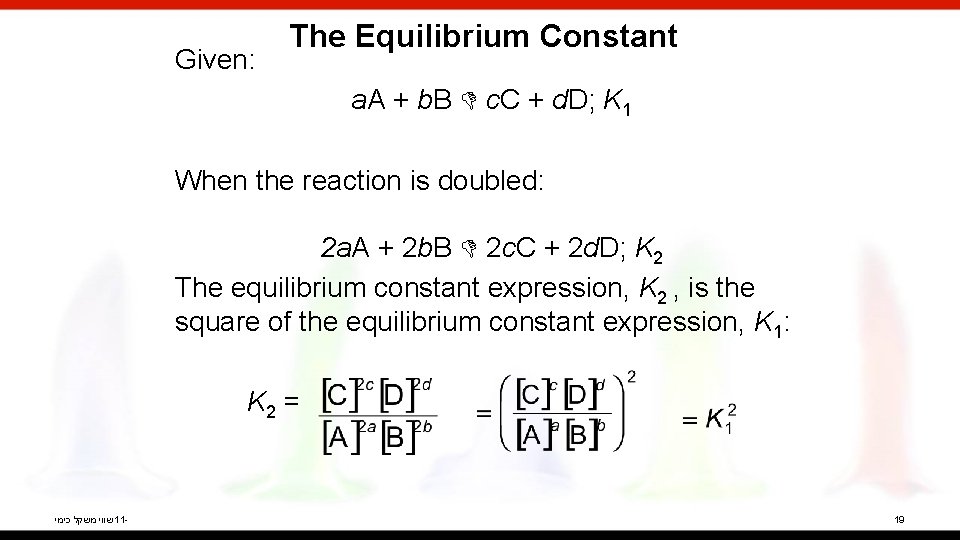
Given: The Equilibrium Constant a. A + b. B c. C + d. D; K 1 When the reaction is doubled: 2 a. A + 2 b. B 2 c. C + 2 d. D; K 2 The equilibrium constant expression, K 2 , is the square of the equilibrium constant expression, K 1: K 2 = שווי משקל כימי 11 - 19
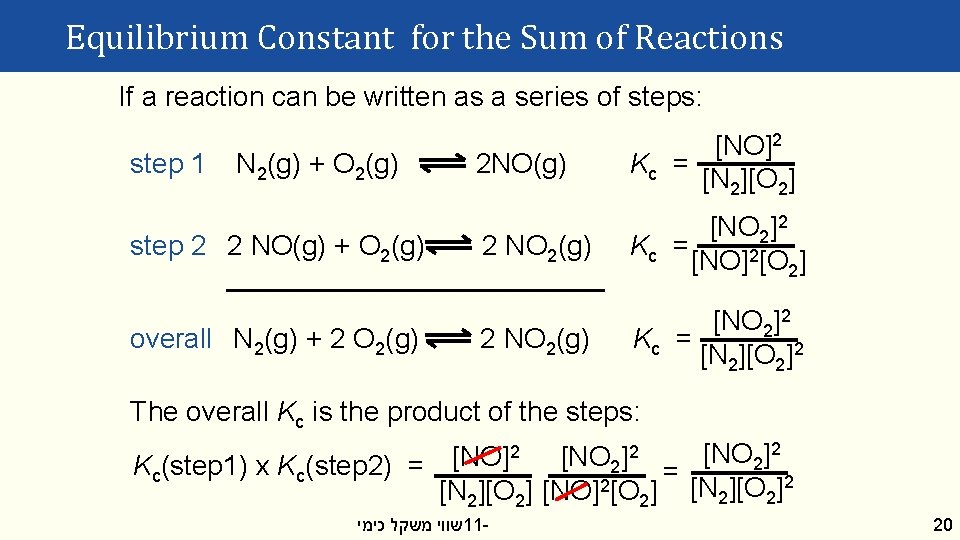
Equilibrium Constant for the Sum of Reactions If a reaction can be written as a series of steps: step 1 N 2(g) + O 2(g) step 2 2 NO(g) + O 2(g) overall N 2(g) + 2 O 2(g) 2 NO(g) [NO]2 Kc = [N 2][O 2] 2 NO 2(g) [NO 2]2 Kc = [NO]2[O 2] 2 NO 2(g) [NO 2]2 Kc = [N 2][O 2]2 The overall Kc is the product of the steps: 2 2 2 [NO ] 2 2 Kc(step 1) x Kc(step 2) = = [N 2][O 2] [NO]2[O 2] [N 2][O 2]2 שווי משקל כימי 11 - 20
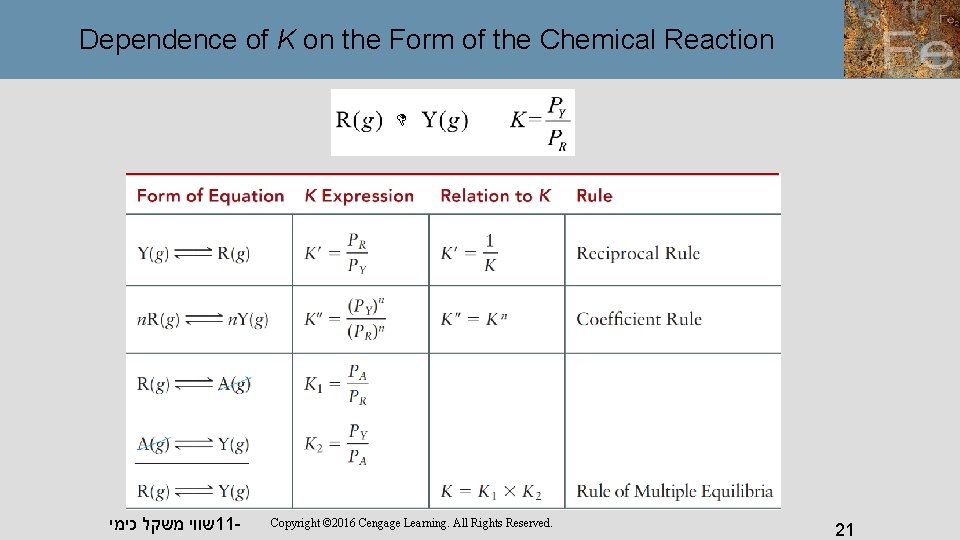
Dependence of K on the Form of the Chemical Reaction שווי משקל כימי 11 - Copyright © 2016 Cengage Learning. All Rights Reserved. 21

The Equilibrium Constant Kp For the reaction a. A(g) + b. B(g) c. C(g) + d. D(g) The equilibrium constant expressions are Kc = שווי משקל כימי 11 - and Kp = 22
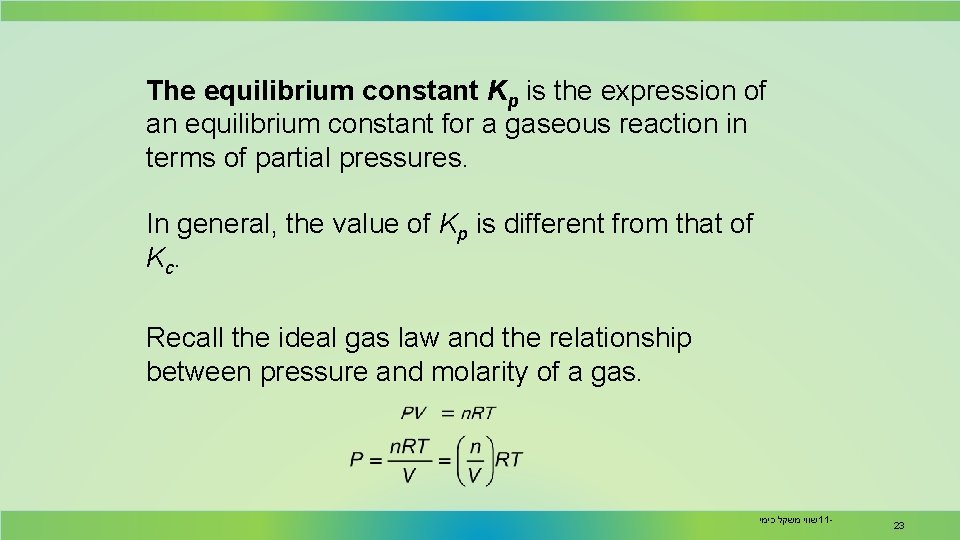
The equilibrium constant Kp is the expression of an equilibrium constant for a gaseous reaction in terms of partial pressures. In general, the value of Kp is different from that of Kc. Recall the ideal gas law and the relationship between pressure and molarity of a gas. שווי משקל כימי 11 - 23

The Equilibrium Constant Kp a. A + b. B c. C + d. D Kp = Kc (RT)Dn שווי משקל כימי 11 - 24

The Equilibrium Constant Kp Consider catalytic methanation. CO(g) + 3 H 2(g) CH 4(g) + H 2 O(g) The equilibrium expression in terms of partial pressures becomes: Kp = Kc (RT)Dn שווי משקל כימי 11 - 25
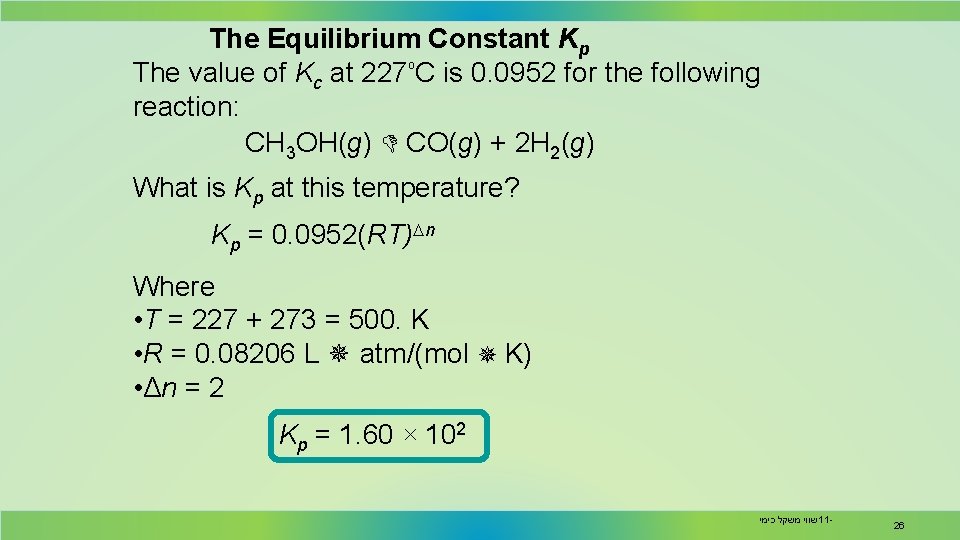
The Equilibrium Constant Kp The value of Kc at 227ₒC is 0. 0952 for the following reaction: CH 3 OH(g) CO(g) + 2 H 2(g) What is Kp at this temperature? Kp = 0. 0952(RT)Dn Where • T = 227 + 273 = 500. K • R = 0. 08206 L atm/(mol K) • Δn = 2 Kp = 1. 60 × 102 שווי משקל כימי 11 - 26
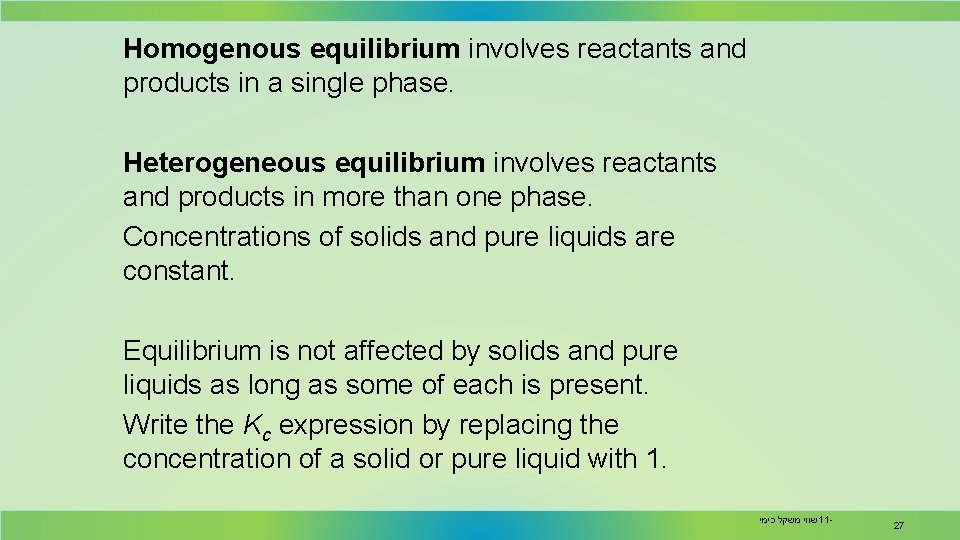
Homogenous equilibrium involves reactants and products in a single phase. Heterogeneous equilibrium involves reactants and products in more than one phase. Concentrations of solids and pure liquids are constant. Equilibrium is not affected by solids and pure liquids as long as some of each is present. Write the Kc expression by replacing the concentration of a solid or pure liquid with 1. שווי משקל כימי 11 - 27
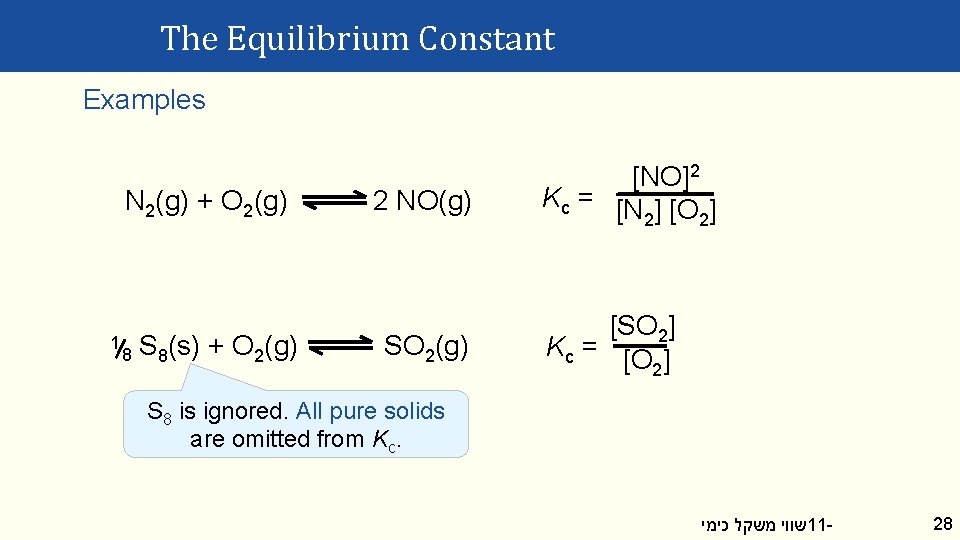
The Equilibrium Constant Examples N 2(g) + O 2(g) 1 8 S 8(s) + O 2(g) 2 NO(g) SO 2(g) [NO]2 Kc = [N ] [O ] 2 2 [SO 2] Kc = [O ] 2 S 8 is ignored. All pure solids are omitted from Kc. שווי משקל כימי 11 - 28
![Equilibria Involving Pure Liquids & Solids [Solid] is constant throughout a reaction. density • Equilibria Involving Pure Liquids & Solids [Solid] is constant throughout a reaction. density •](http://slidetodoc.com/presentation_image_h/80c4bf340b5680d3664d2abc22305c71/image-29.jpg)
Equilibria Involving Pure Liquids & Solids [Solid] is constant throughout a reaction. density • pure solid concentration = mol. wt g/L g / mol • [S 8] = d. S 8 / mol. wt S 8 • d and mol. wt. are constants, so [S 8] is constant. • This constant factor is “absorbed” into Kc. Pure liquids are omitted for the same reason. שווי משקל כימי 11 - 29
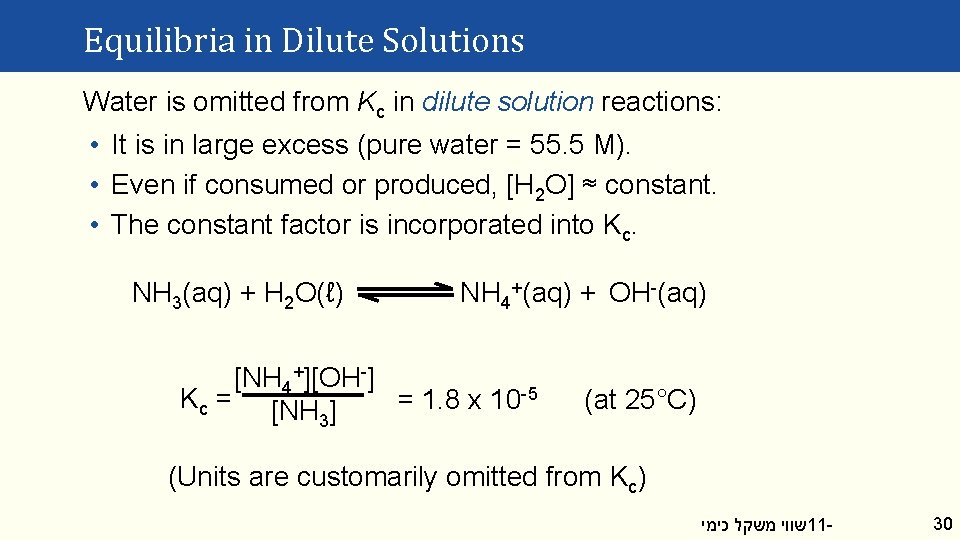
Equilibria in Dilute Solutions Water is omitted from Kc in dilute solution reactions: • It is in large excess (pure water = 55. 5 M). • Even if consumed or produced, [H 2 O] ≈ constant. • The constant factor is incorporated into Kc. NH 3(aq) + H 2 O(ℓ) NH 4+(aq) + OH-(aq) [NH 4+][OH-] Kc = [NH ] = 1. 8 x 10 -5 3 (at 25°C) (Units are customarily omitted from Kc) שווי משקל כימי 11 - 30

Meaning of the Equilibrium Constant When: Kc >> 1 Reaction is strongly product favored. • very little reactant remains. • often written as a forward reaction only. • assume reaction goes to completion. Kc << 1 Reaction is strongly reactant favored. • very little product forms. • usually written as “no reaction” or NR. Kc ≈ 1 Reactants & products present at equilibrium. • use equilibrium methods discussed here. שווי משקל כימי 11 - 31
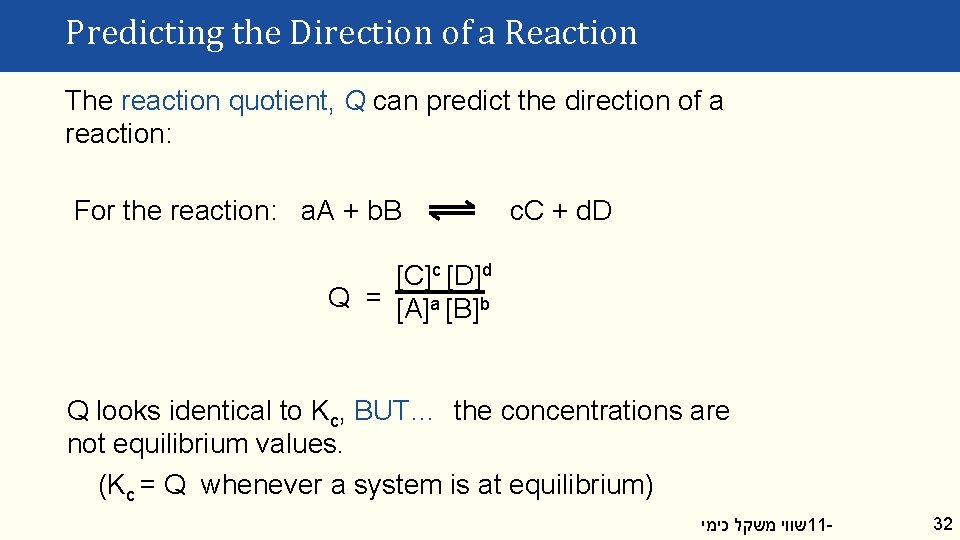
Predicting the Direction of a Reaction The reaction quotient, Q can predict the direction of a reaction: For the reaction: a. A + b. B c. C + d. D [C]c [D]d Q = [A]a [B]b Q looks identical to Kc, BUT… the concentrations are not equilibrium values. (Kc = Q whenever a system is at equilibrium) שווי משקל כימי 11 - 32
![Predicting the Direction of a Reaction [Products] Q = [Reactants] Changes as a reaction Predicting the Direction of a Reaction [Products] Q = [Reactants] Changes as a reaction](http://slidetodoc.com/presentation_image_h/80c4bf340b5680d3664d2abc22305c71/image-33.jpg)
Predicting the Direction of a Reaction [Products] Q = [Reactants] Changes as a reaction moves to equilibrium [Products]equilib Kc= [Reactants] equilib Constant! (If T is constant) If Q < Kc, Q must increase to reach equilibrium. • make more product (and less reactant). • move forward. If Q > Kc, Q must decrease to reach equilibrium. • make less product (and more reactant). • move back. שווי משקל כימי 11 - 33
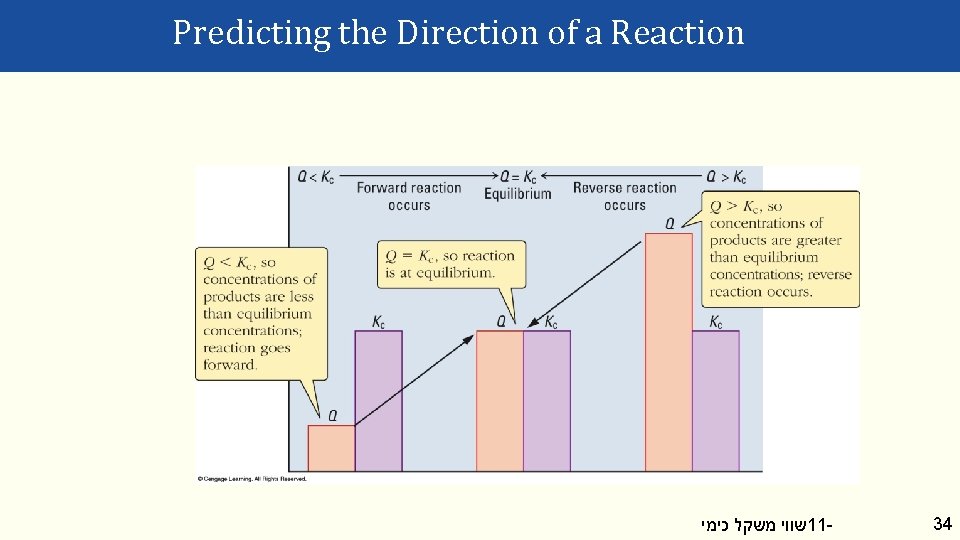
Predicting the Direction of a Reaction שווי משקל כימי 11 - 34
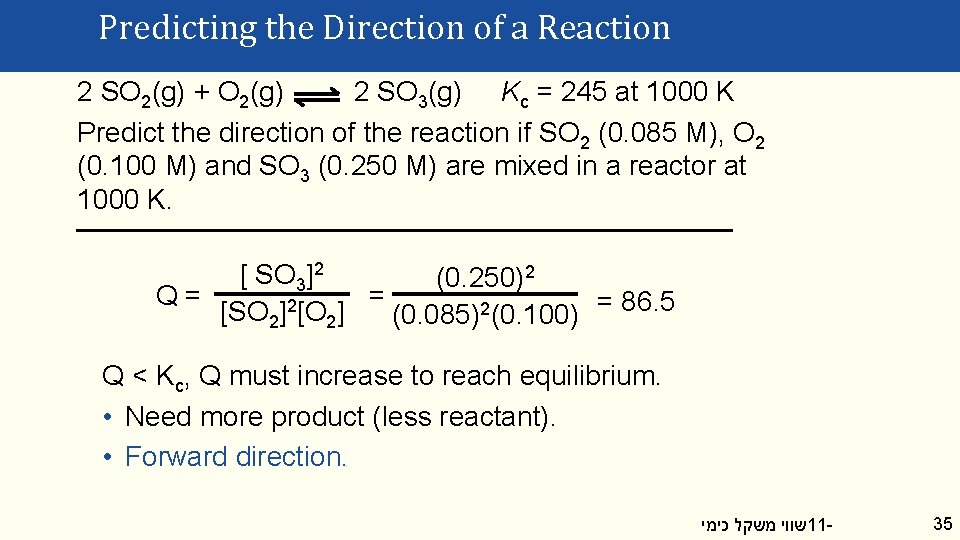
Predicting the Direction of a Reaction 2 SO 2(g) + O 2(g) 2 SO 3(g) Kc = 245 at 1000 K Predict the direction of the reaction if SO 2 (0. 085 M), O 2 (0. 100 M) and SO 3 (0. 250 M) are mixed in a reactor at 1000 K. [ SO 3]2 (0. 250)2 Q= = 2 [SO 2] [O 2] (0. 085)2(0. 100) = 86. 5 Q < Kc, Q must increase to reach equilibrium. • Need more product (less reactant). • Forward direction. שווי משקל כימי 11 - 35
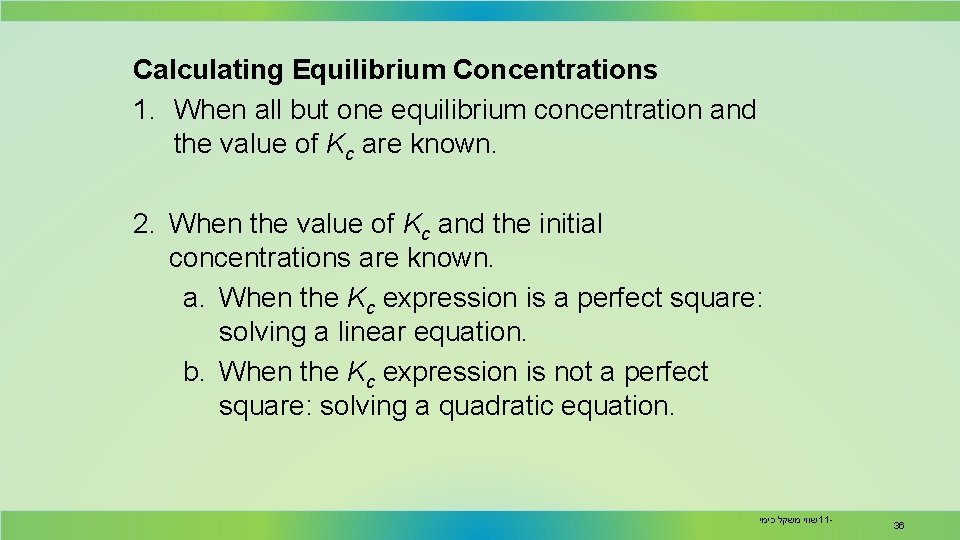
Calculating Equilibrium Concentrations 1. When all but one equilibrium concentration and the value of Kc are known. 2. When the value of Kc and the initial concentrations are known. a. When the Kc expression is a perfect square: solving a linear equation. b. When the Kc expression is not a perfect square: solving a quadratic equation. שווי משקל כימי 11 - 36
Obtaining One Equilibrium Concentration Given the Others A gaseous mixture contains 0. 30 mol CO, 0. 10 mol H 2, and 0. 020 mol H 2 O, plus an unknown amount of CH 4, in each liter. This mixture is at equilibrium at 1200 K. CO(g) + 3 H 2(g) CH 4(g) + H 2 O(g) What is the concentration of CH 4 in this mixture? The equilibrium constant Kc equals 3. 92. שווי משקל כימי 11 - 37
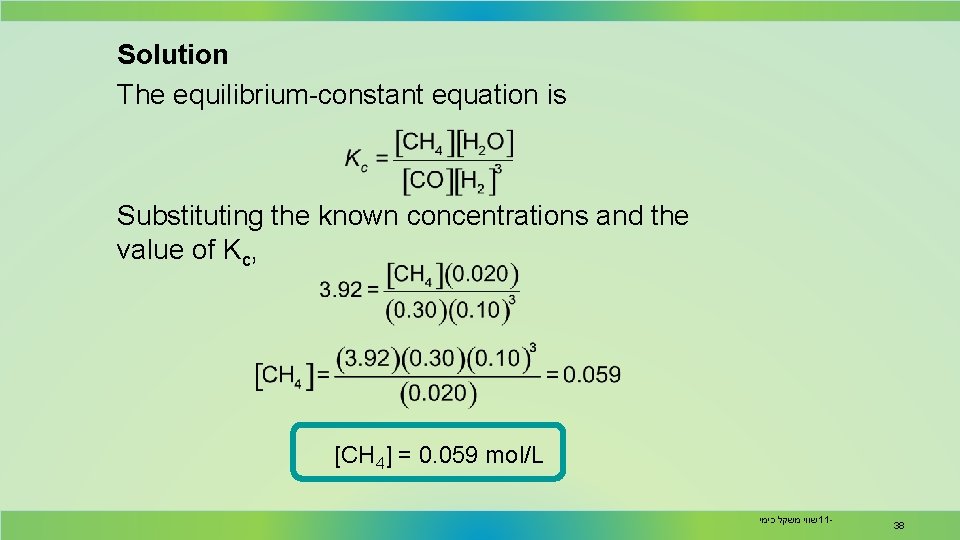
Solution The equilibrium-constant equation is Substituting the known concentrations and the value of Kc, [CH 4] = 0. 059 mol/L שווי משקל כימי 11 - 38
Solving an Equilibrium Problem ( Involving a Linear Equation in X) The following reaction is used to increase the ratio of hydrogen in synthesis gas (mixtures of CO and H 2). CO(g) + H 2 O(g) CO 2(g) +H 2(g) If you start with 1. 00 mol each of carbon monoxide and water in a 50. 0 -L vessel, how many moles of each substance are in the equilibrium mixture at ₒ 1000 C? Kc at this temperature is 0. 58. שווי משקל כימי 11 - 39

Solution Step 1 The starting concentrations of CO and H 2 O are The starting concentrations of the products CO 2 and H 2 are 0. The change in concentration when the mixture goes to equilibrium are not given. They can be written in terms of a single unknown. שווי משקל כימי 11 - 40

Solution Let x be the moles of CO 2 formed per liter. The moles of H 2 formed per liter is also x. Similarly x moles of each CO and H 2 O are consumed. The changes for these can be written as – x. The equilibrium concentrations can be obtained by adding the change in concentration to the starting concentrations. שווי משקל כימי 11 - 41
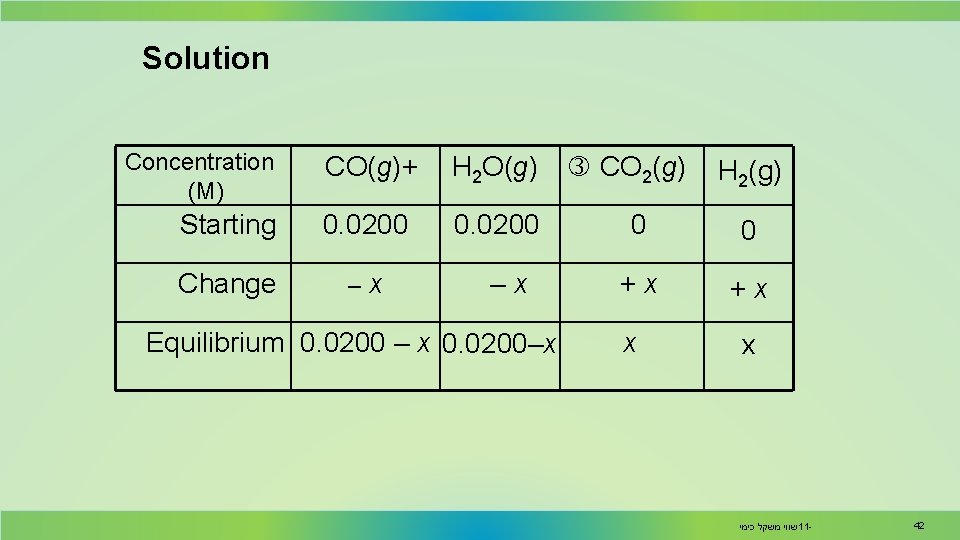
Solution Concentration (M) CO(g)+ H 2 O(g) Starting 0. 0200 Change –x –x Equilibrium 0. 0200 – x 0. 0200–x CO 2(g) H 2(g) 0 0 +x +x x x שווי משקל כימי 11 - 42
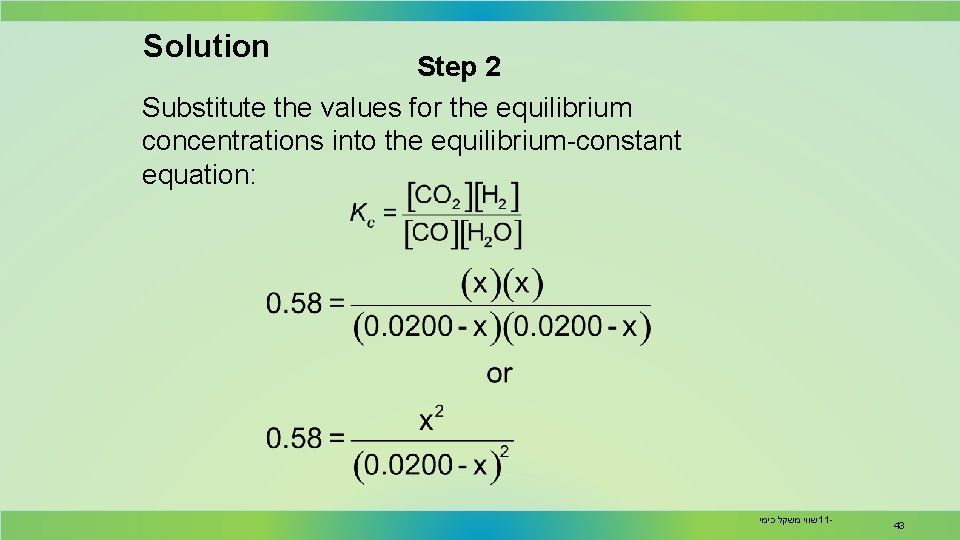
Solution Step 2 Substitute the values for the equilibrium concentrations into the equilibrium-constant equation: שווי משקל כימי 11 - 43
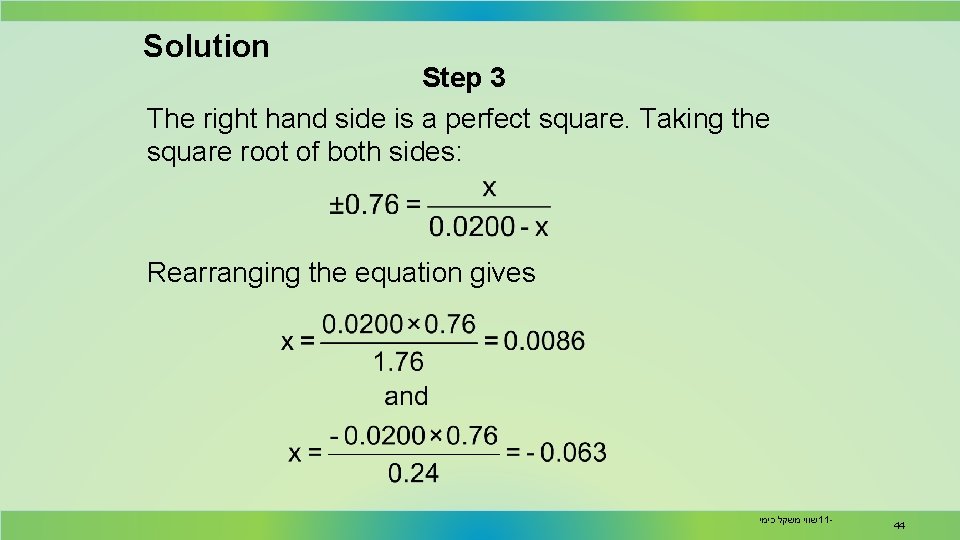
Solution Step 3 The right hand side is a perfect square. Taking the square root of both sides: Rearranging the equation gives שווי משקל כימי 11 - 44
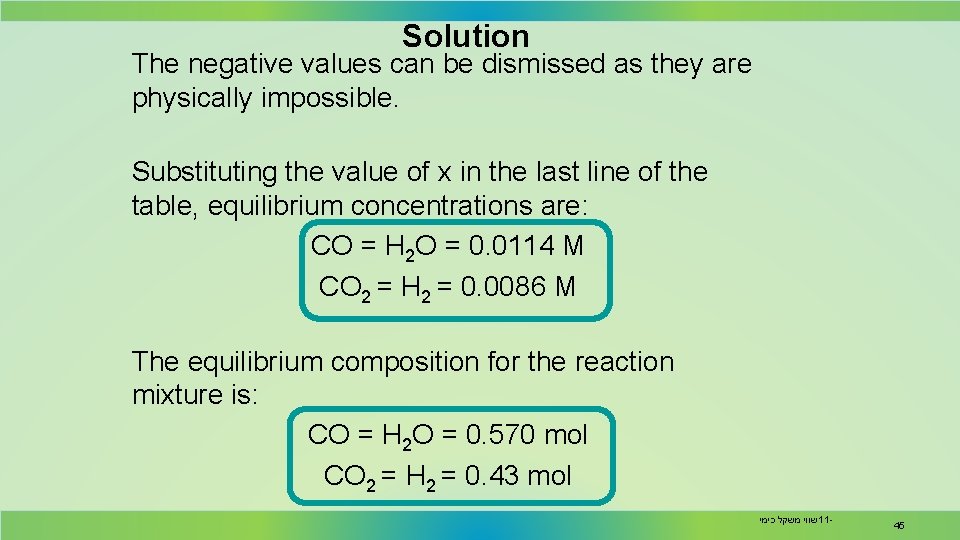
Solution The negative values can be dismissed as they are physically impossible. Substituting the value of x in the last line of the table, equilibrium concentrations are: CO = H 2 O = 0. 0114 M CO 2 = H 2 = 0. 0086 M The equilibrium composition for the reaction mixture is: CO = H 2 O = 0. 570 mol CO 2 = H 2 = 0. 43 mol שווי משקל כימי 11 - 45
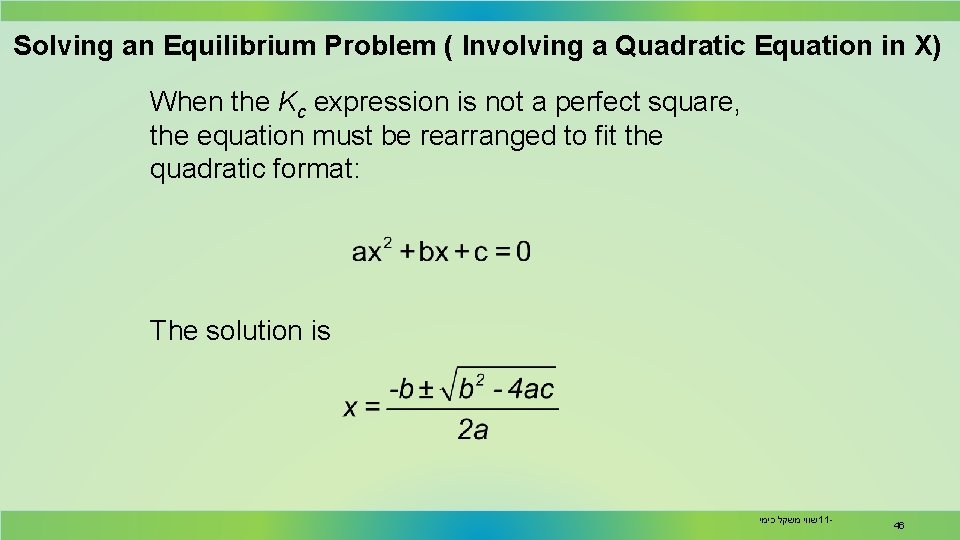
Solving an Equilibrium Problem ( Involving a Quadratic Equation in X) When the Kc expression is not a perfect square, the equation must be rearranged to fit the quadratic format: The solution is שווי משקל כימי 11 - 46
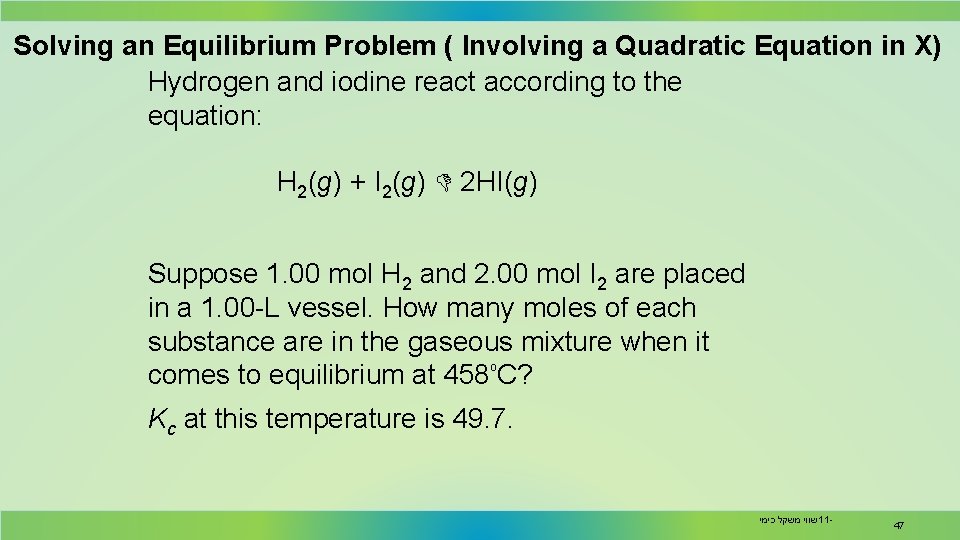
Solving an Equilibrium Problem ( Involving a Quadratic Equation in X) Hydrogen and iodine react according to the equation: H 2(g) + I 2(g) 2 HI(g) Suppose 1. 00 mol H 2 and 2. 00 mol I 2 are placed in a 1. 00 -L vessel. How many moles of each substance are in the gaseous mixture when it ₒ comes to equilibrium at 458 C? Kc at this temperature is 49. 7. שווי משקל כימי 11 - 47
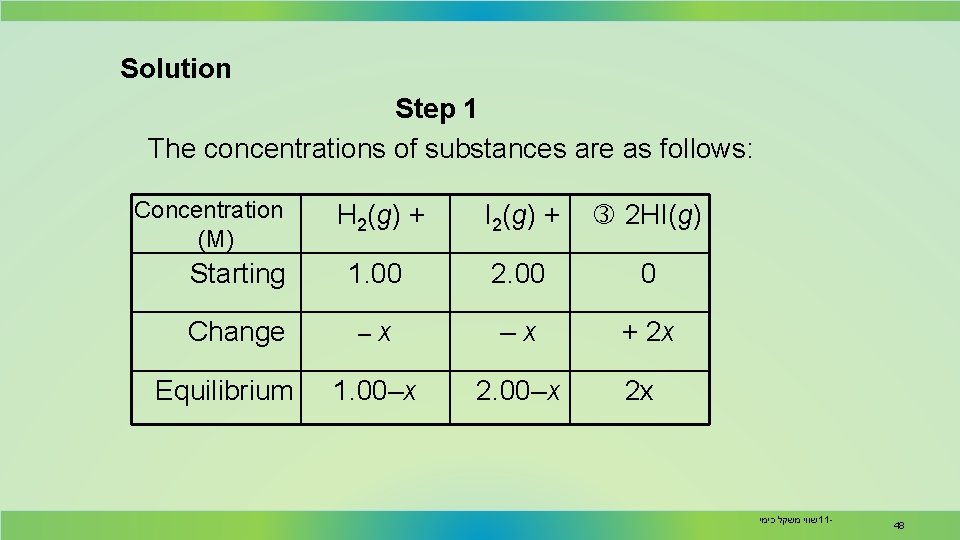
Solution Step 1 The concentrations of substances are as follows: Concentration (M) H 2 (g ) + I 2 (g ) + 2 HI(g) Starting 1. 00 2. 00 0 Change –x –x + 2 x 1. 00–x 2. 00–x Equilibrium 2 x שווי משקל כימי 11 - 48
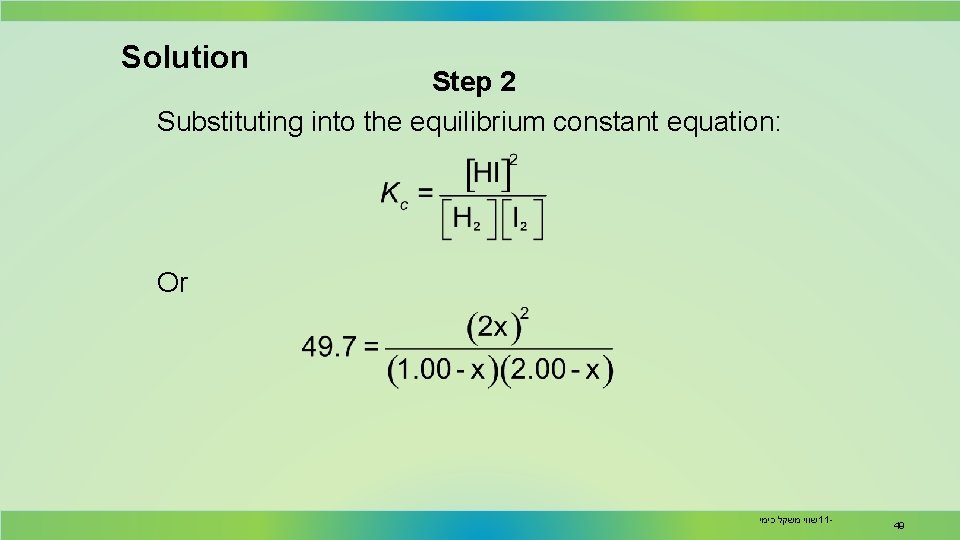
Solution Step 2 Substituting into the equilibrium constant equation: Or שווי משקל כימי 11 - 49
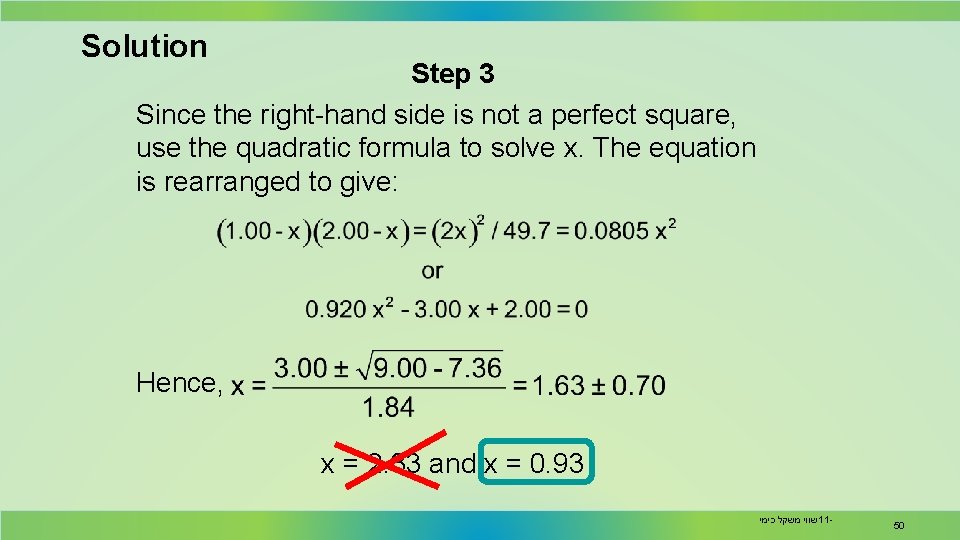
Solution Step 3 Since the right-hand side is not a perfect square, use the quadratic formula to solve x. The equation is rearranged to give: Hence, x = 2. 33 and x = 0. 93 שווי משקל כימי 11 - 50
Solution Since x = 2. 33 gives a negative value to 1. 00 – x, use x = 0. 93 while substituting. Equilibrium composition of the substances are as follows: H 2 = 0. 07 mol (1. 00 – 0. 93) I 2 = 1. 07 mol (2. 00 – 0. 93) HI = 1. 86 mol (2 x) שווי משקל כימי 11 - 51
Le Châtelier’s Principle When a system in chemical equilibrium is disturbed by a change in: • Temperature • Pressure • Concentration The system shifts in equilibrium composition in a way that tends to counteract this change of variable. שווי משקל כימי 11 - 52

Le Châtelier’s Principle When more reactant is added to, or some product is removed from, an equilibrium mixture, net reaction occurs left to right (forward direction) to give new equilibrium and produce more products. When more product is added to, or some reactant is removed from, an equilibrium mixture, net reaction occurs from right to left (reverse direction) to give new equilibrium and produce more reactants. שווי משקל כימי 11 - 53
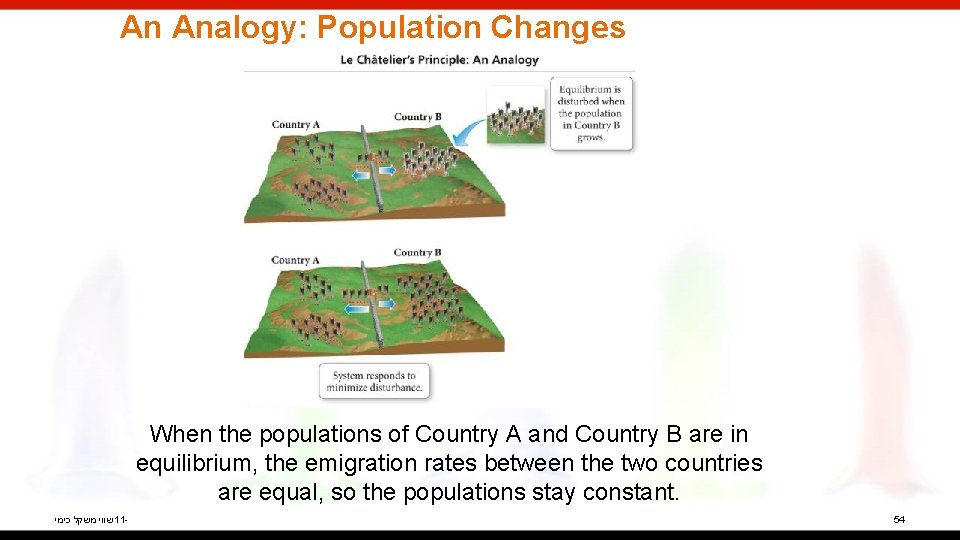
An Analogy: Population Changes When the populations of Country A and Country B are in equilibrium, the emigration rates between the two countries are equal, so the populations stay constant. שווי משקל כימי 11 - 54
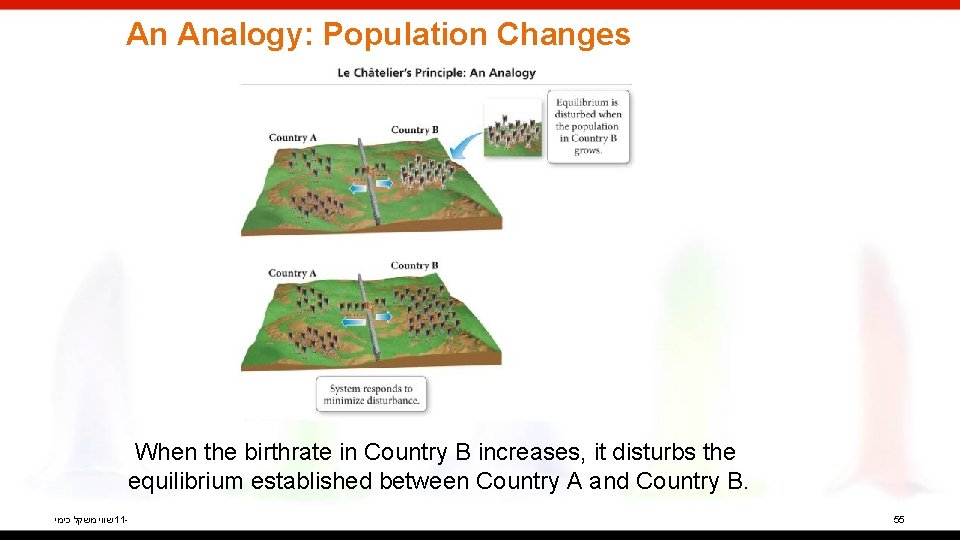
An Analogy: Population Changes When the birthrate in Country B increases, it disturbs the equilibrium established between Country A and Country B. שווי משקל כימי 11 - 55
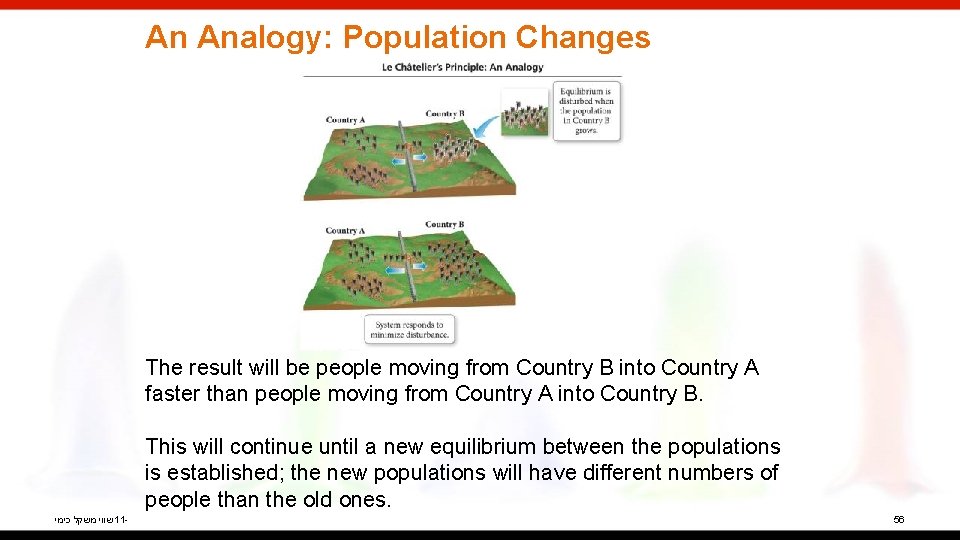
An Analogy: Population Changes The result will be people moving from Country B into Country A faster than people moving from Country A into Country B. This will continue until a new equilibrium between the populations is established; the new populations will have different numbers of people than the old ones. שווי משקל כימי 11 - 56
Applying Le Châtelier’s Principle When a Concentration Is Altered Predict the direction of reaction when H 2 is removed from a mixture (lowering its concentration) in which the following equilibrium has been established: H 2(g) + I 2(g) 2 HI(g) Solution When H 2 is removed, the reaction goes in reverse direction to partially restore the removed substance. שווי משקל כימי 11 - 57
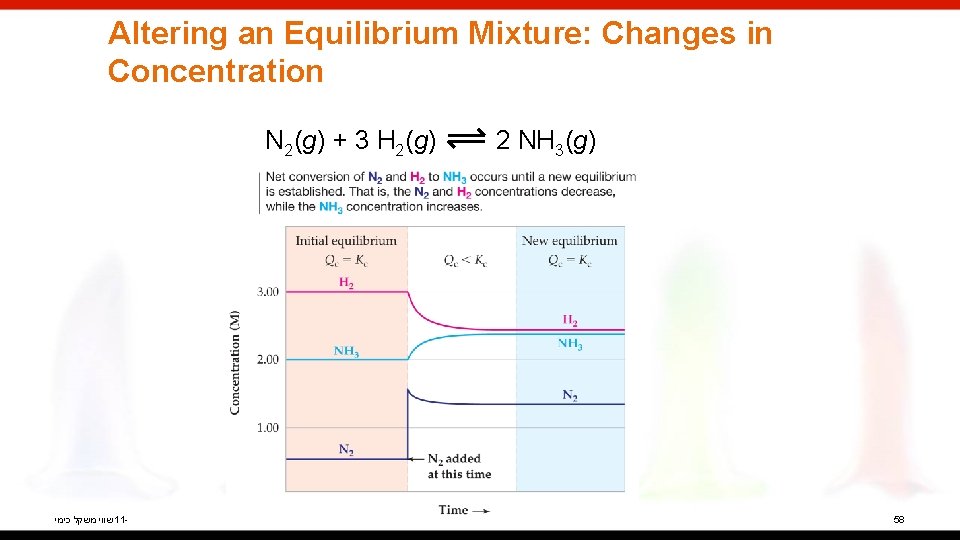
Altering an Equilibrium Mixture: Changes in Concentration N 2(g) + 3 H 2(g) שווי משקל כימי 11 - 2 NH 3(g) 58
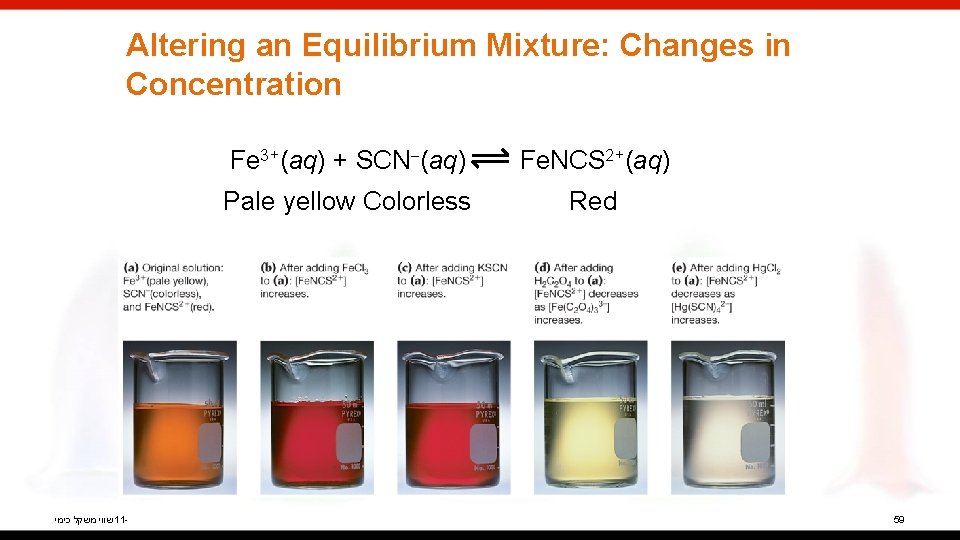
Altering an Equilibrium Mixture: Changes in Concentration שווי משקל כימי 11 - Fe 3+(aq) + SCN (aq) Fe. NCS 2+(aq) Pale yellow Colorless Red 59
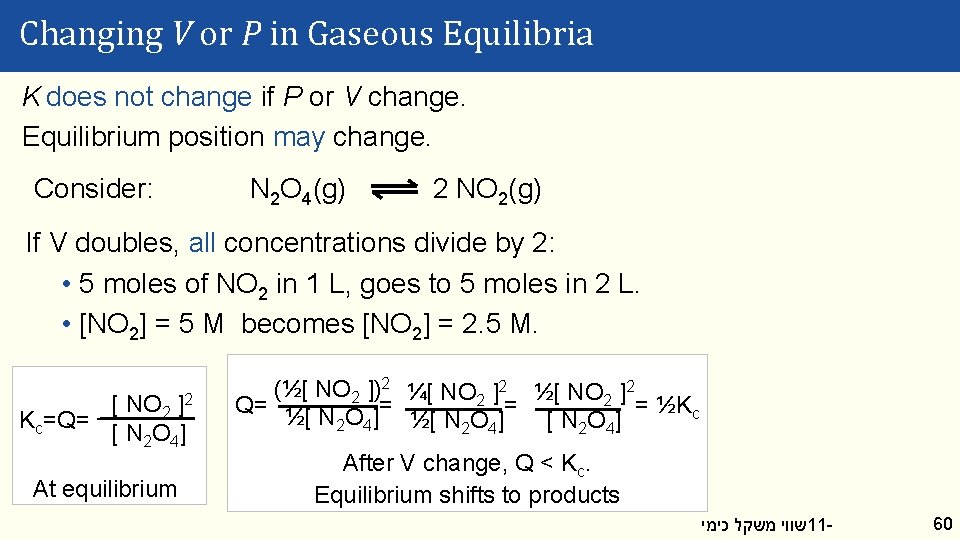
Changing V or P in Gaseous Equilibria K does not change if P or V change. Equilibrium position may change. Consider: N 2 O 4(g) 2 NO 2(g) If V doubles, all concentrations divide by 2: • 5 moles of NO 2 in 1 L, goes to 5 moles in 2 L. • [NO 2] = 5 M becomes [NO 2] = 2. 5 M. [ NO 2 ]2 Kc=Q= [ N 2 O 4] At equilibrium (½[ NO 2 ])2 ¼[ NO 2 ]2 ½[ NO 2 ]2 Q= = ½Kc ½[ N 2 O 4] After V change, Q < Kc. Equilibrium shifts to products שווי משקל כימי 11 - 60

Changing V or P in Gaseous Equilibria This is consistent with Le Chatelier: N 2 O 4(g) 2 NO 2(g) V doubled = lower concentration. Minimize change by: • Making more molecules (increase concentration). • Shifting toward products; convert 1 molecule into 2. P doubled. Minimize the change by: • Removing molecules (decrease P). • Shifting toward reactants; convert 2 molecules into 1. שווי משקל כימי 11 - 61
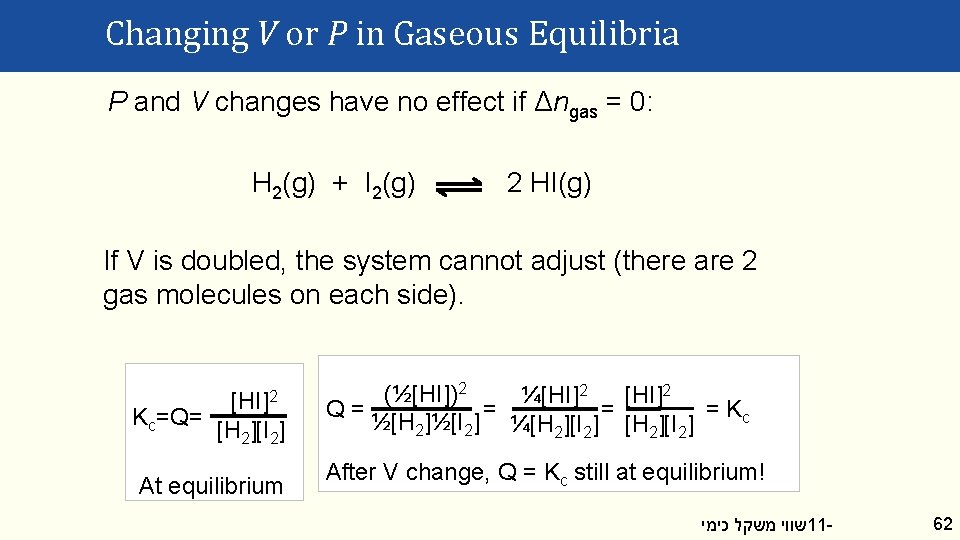
Changing V or P in Gaseous Equilibria P and V changes have no effect if Δngas = 0: H 2(g) + I 2(g) 2 HI(g) If V is doubled, the system cannot adjust (there are 2 gas molecules on each side). Kc=Q= [HI]2 [H 2][I 2] At equilibrium (½[HI])2 ¼[HI]2 Q= = Kc ½[H 2]½[I 2] ¼[H 2][I 2] After V change, Q = Kc still at equilibrium! שווי משקל כימי 11 - 62
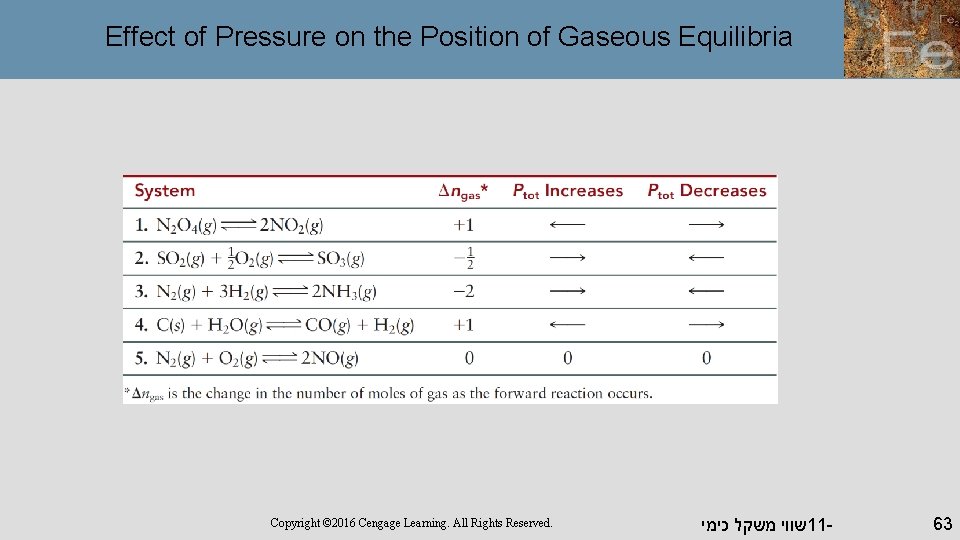
Effect of Pressure on the Position of Gaseous Equilibria Copyright © 2016 Cengage Learning. All Rights Reserved. שווי משקל כימי 11 - 63
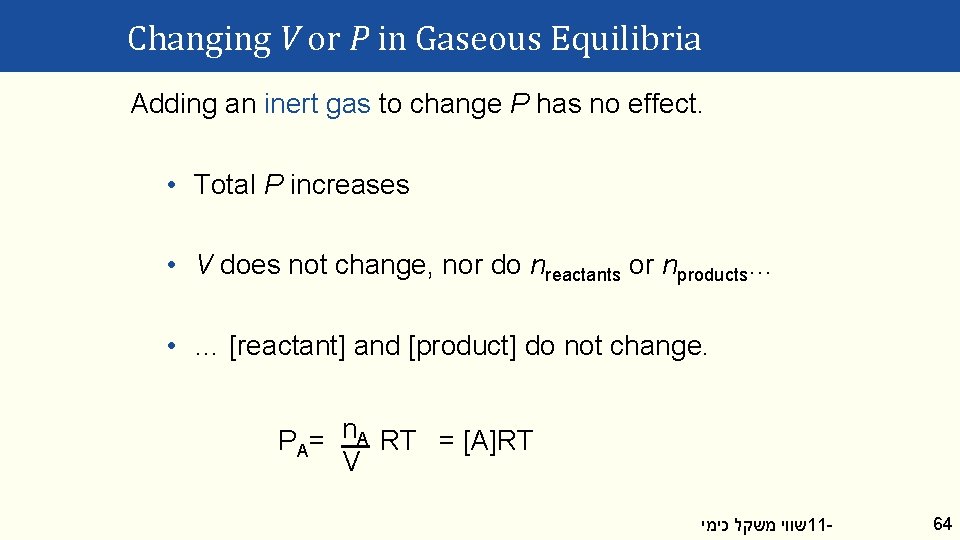
Changing V or P in Gaseous Equilibria Adding an inert gas to change P has no effect. • Total P increases • V does not change, nor do nreactants or nproducts… • … [reactant] and [product] do not change. PA= n. A RT = [A]RT V שווי משקל כימי 11 - 64
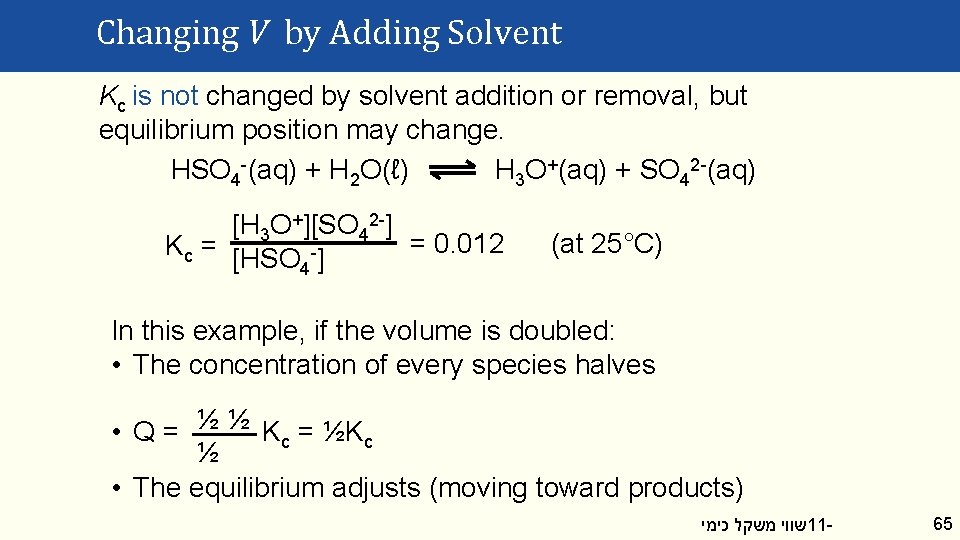
Changing V by Adding Solvent Kc is not changed by solvent addition or removal, but equilibrium position may change. HSO 4 -(aq) + H 2 O(ℓ) H 3 O+(aq) + SO 42 -(aq) [H 3 O+][SO 42 -] = 0. 012 Kc = [HSO -] 4 (at 25°C) In this example, if the volume is doubled: • The concentration of every species halves • Q = ½ ½ Kc = ½Kc ½ • The equilibrium adjusts (moving toward products) שווי משקל כימי 11 - 65
Effect of Temperature Change • Reaction rates increase with increase in temperature. • Changing the temperature changes the value of the equilibrium constant and also causes a shift in the equilibrium. • The direction of each of these changes depends on the sign of ΔHₒ. שווי משקל כימי 11 - 66
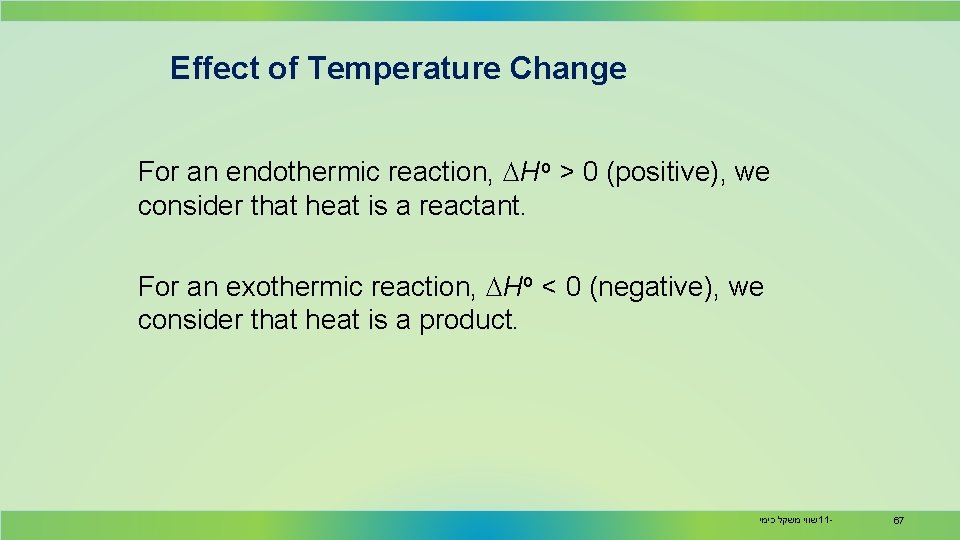
Effect of Temperature Change For an endothermic reaction, DHo > 0 (positive), we consider that heat is a reactant. For an exothermic reaction, DHo < 0 (negative), we consider that heat is a product. שווי משקל כימי 11 - 67
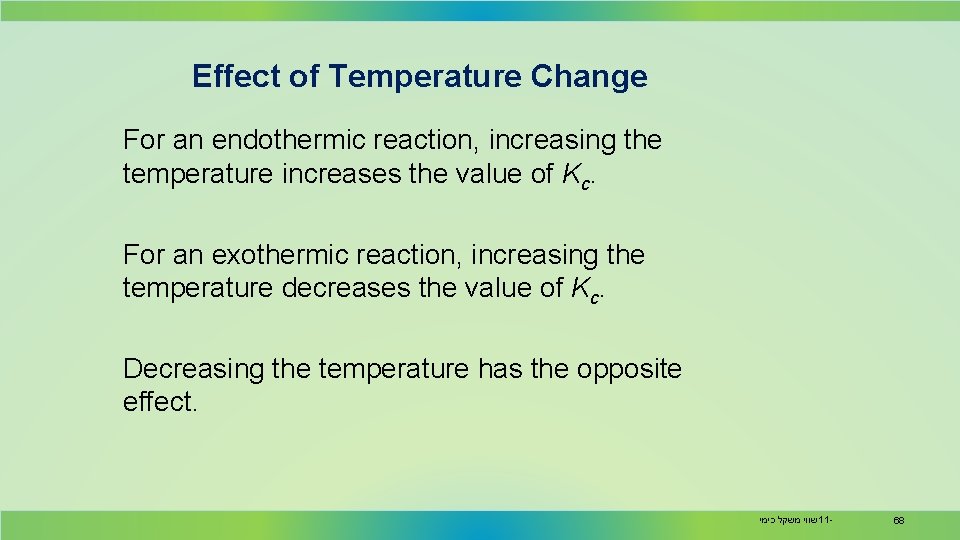
Effect of Temperature Change For an endothermic reaction, increasing the temperature increases the value of Kc. For an exothermic reaction, increasing the temperature decreases the value of Kc. Decreasing the temperature has the opposite effect. שווי משקל כימי 11 - 68
Effect of Temperature Change Given: 2 H 2 O(g) 2 H 2(g) + O 2(g); DH° = 484 k. J Would you expect this reaction to be favorable at high or low temperatures? We rewrite the reaction to include heat: Heat + 2 H 2 O(g) 2 H 2(g) + O 2(g) When heat is added, the reaction shifts forward = right = . The reaction is favorable at high temperatures. שווי משקל כימי 11 - 69

Altering an Equilibrium Mixture: Changes in Temperature N 2 O 4(g) Colorless שווי משקל כימי 11 - 2 NO 2(g) Brown 70
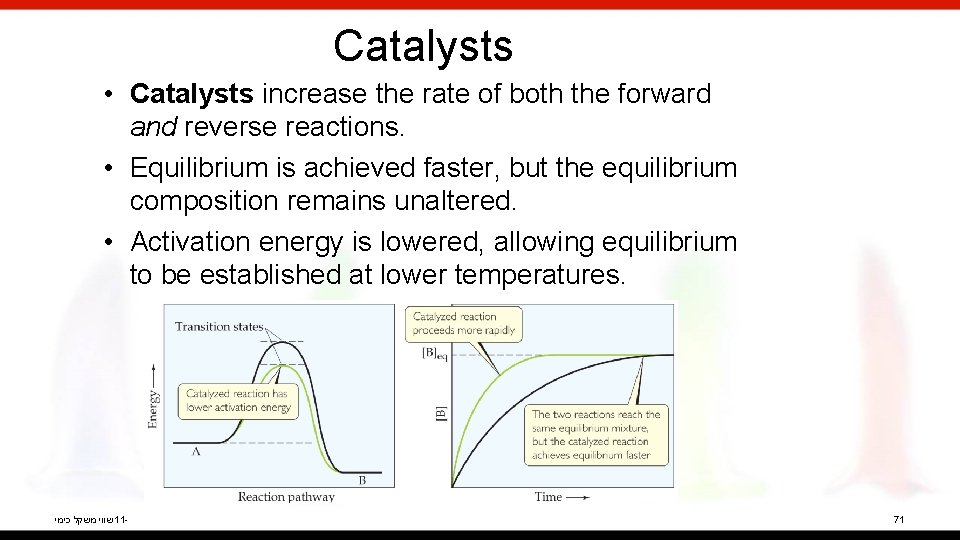
Catalysts • Catalysts increase the rate of both the forward and reverse reactions. • Equilibrium is achieved faster, but the equilibrium composition remains unaltered. • Activation energy is lowered, allowing equilibrium to be established at lower temperatures. שווי משקל כימי 11 - 71
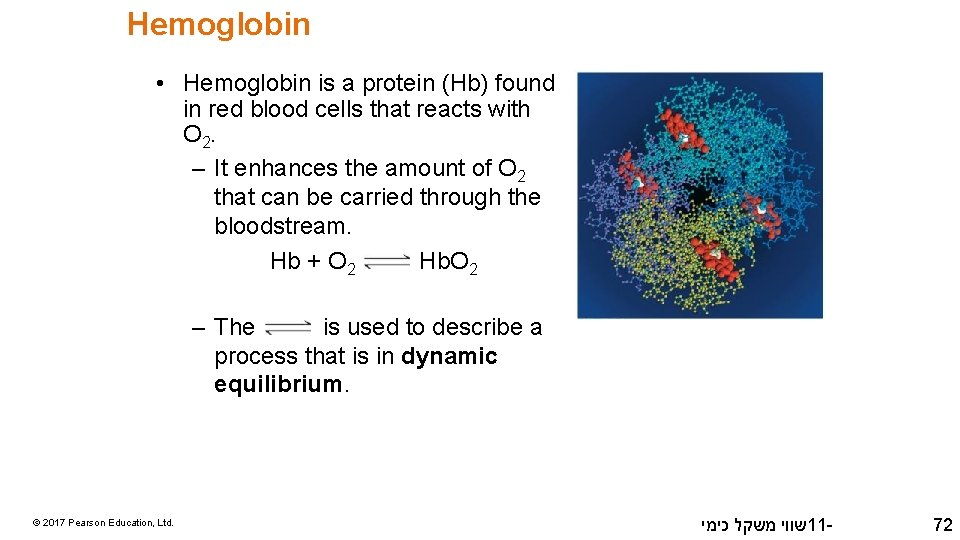
Hemoglobin • Hemoglobin is a protein (Hb) found in red blood cells that reacts with O 2. – It enhances the amount of O 2 that can be carried through the bloodstream. Hb + O 2 Hb. O 2 – The is used to describe a process that is in dynamic equilibrium. © 2017 Pearson Education, Ltd. שווי משקל כימי 11 - 72
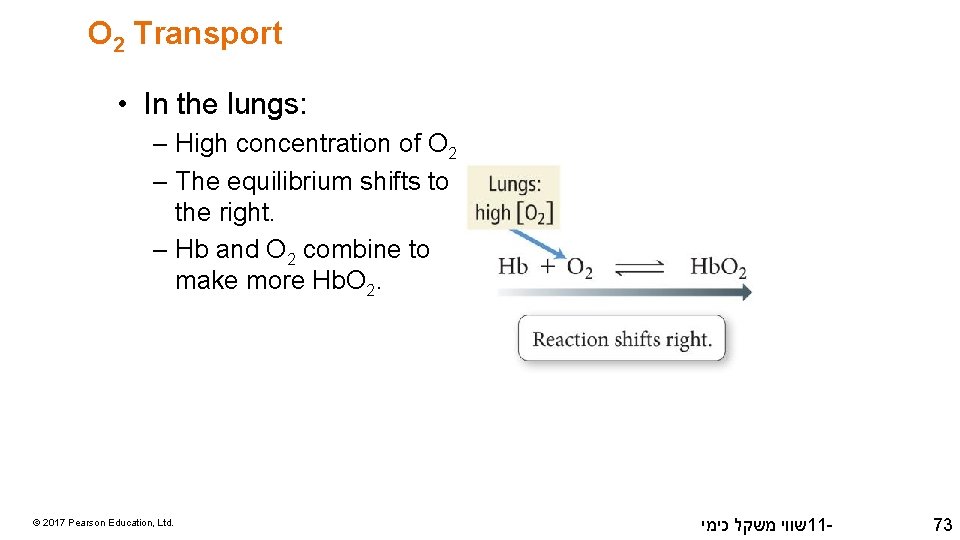
O 2 Transport • In the lungs: – High concentration of O 2 – The equilibrium shifts to the right. – Hb and O 2 combine to make more Hb. O 2. © 2017 Pearson Education, Ltd. שווי משקל כימי 11 - 73
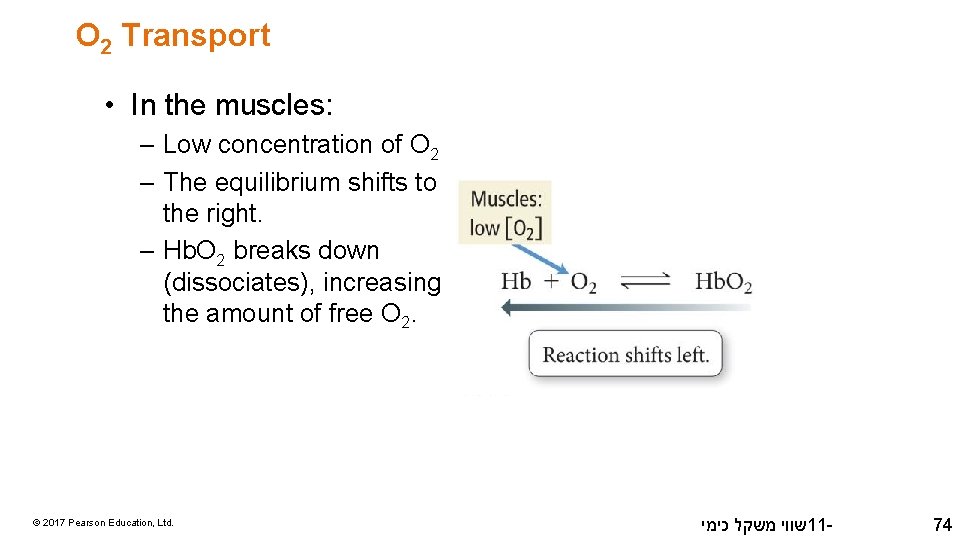
O 2 Transport • In the muscles: – Low concentration of O 2 – The equilibrium shifts to the right. – Hb. O 2 breaks down (dissociates), increasing the amount of free O 2. © 2017 Pearson Education, Ltd. שווי משקל כימי 11 - 74
Fetal Hemoglobin • Fetal hemoglobin’s equilibrium constant is larger than adult hemoglobin’s constant. • Fetal hemoglobin is more efficient at binding O 2. • O 2 is transferred to the fetal hemoglobin from the mother’s hemoglobin in the placenta. © 2017 Pearson Education, Ltd. שווי משקל כימי 11 - 75
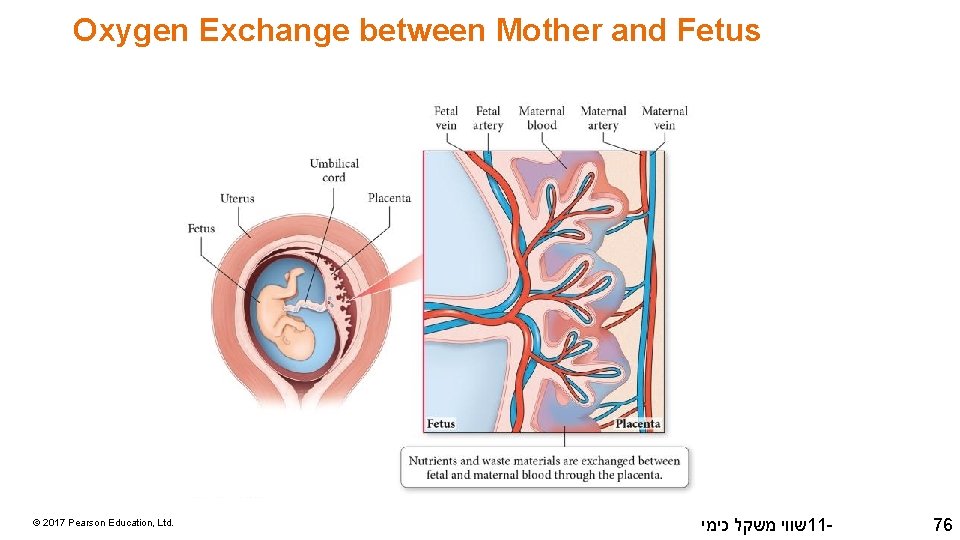
Oxygen Exchange between Mother and Fetus © 2017 Pearson Education, Ltd. שווי משקל כימי 11 - 76
- Slides: 76