CHEMICAL EQUILIBRIUM Chapter 18 REVERSIBLE REACTIONS Every reaction

CHEMICAL EQUILIBRIUM Chapter 18
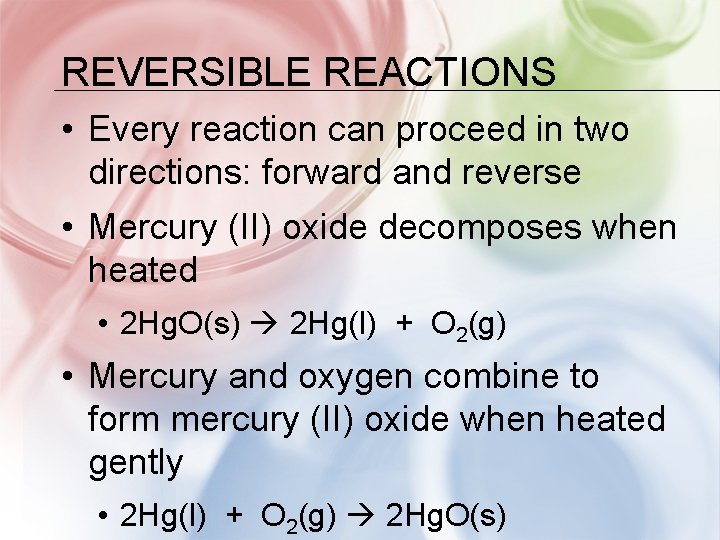
REVERSIBLE REACTIONS • Every reaction can proceed in two directions: forward and reverse • Mercury (II) oxide decomposes when heated • 2 Hg. O(s) 2 Hg(l) + O 2(g) • Mercury and oxygen combine to form mercury (II) oxide when heated gently • 2 Hg(l) + O 2(g) 2 Hg. O(s)

CHEMICAL EQUILIBRIUM • When the rate of its forward reaction equals the rate of its reverse reaction

HBr(aq) + H 2 O(l) H 3 O+(aq) + Br-(aq) • In some cases, the forward reaction is nearly completed before the rate of the reverse reaction is high enough to establish equilibrium • Reaction to the right • Higher concentration of products
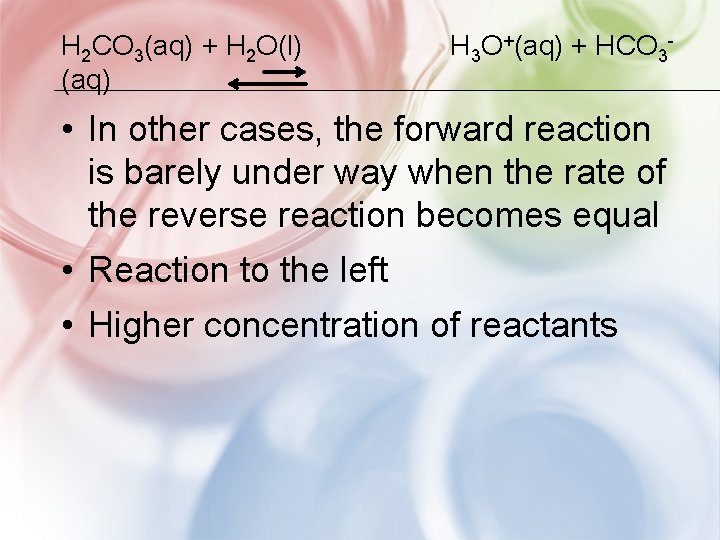
H 2 CO 3(aq) + H 2 O(l) (aq) H 3 O+(aq) + HCO 3 - • In other cases, the forward reaction is barely under way when the rate of the reverse reaction becomes equal • Reaction to the left • Higher concentration of reactants

H 2 SO 3(aq) + H 2 O(l) (aq) H 3 O+(aq) + HSO 3 - • When neither reaction is favored • Considerable concentration of both reactants and products at equilibrium • Chemists try to convert as much of reactants as possible • How much reactants into products can be determined from the equilibrium constant
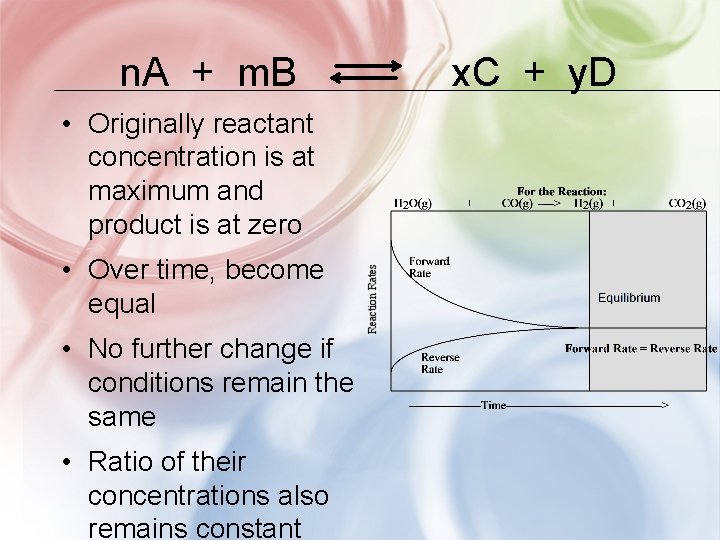
n. A + m. B • Originally reactant concentration is at maximum and product is at zero • Over time, become equal • No further change if conditions remain the same • Ratio of their concentrations also remains constant x. C + y. D

n. A + m. B x. C + y. D • Equilibrium constant (K) K = [C]x[D]y [A]n[B]m
![H 2(g) + I 2(g) • Equilibrium constant (K) K= [HI]2 [H 2][I 2] H 2(g) + I 2(g) • Equilibrium constant (K) K= [HI]2 [H 2][I 2]](http://slidetodoc.com/presentation_image_h2/211fe2cafad6f3c40045ff012e6c55b2/image-9.jpg)
H 2(g) + I 2(g) • Equilibrium constant (K) K= [HI]2 [H 2][I 2] 2 HI(g)
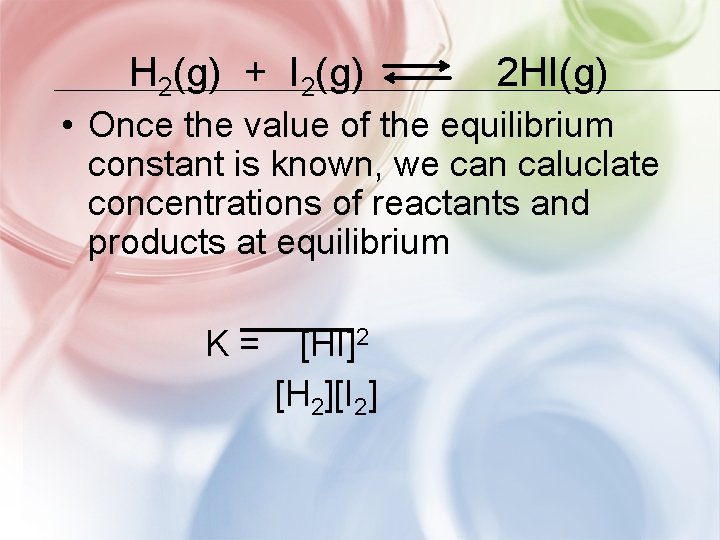
H 2(g) + I 2(g) 2 HI(g) • Once the value of the equilibrium constant is known, we can caluclate concentrations of reactants and products at equilibrium K= [HI]2 [H 2][I 2]
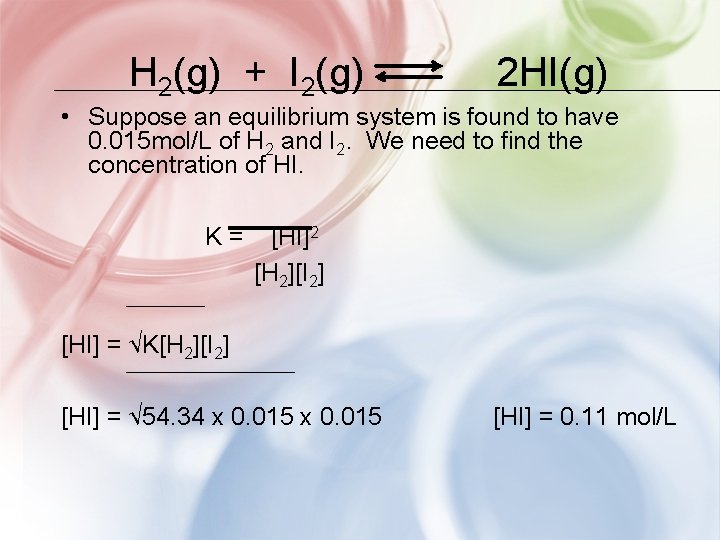
H 2(g) + I 2(g) 2 HI(g) • Suppose an equilibrium system is found to have 0. 015 mol/L of H 2 and I 2. We need to find the concentration of HI. K= [HI]2 [H 2][I 2] [HI] = √K[H 2][I 2] [HI] = √ 54. 34 x 0. 015 [HI] = 0. 11 mol/L
- Slides: 11