CHEMICAL EQUILIBRIUM Chapter 18 I will discover that

CHEMICAL EQUILIBRIUM Chapter 18 I will discover that many reactions and processes reach a state of chemical equilibrium. I will use Le Chatelier’s Principle to explain how various factors affect chemical equilibrium. I will calculate equilibrium concentrations of reactants and products using the equilibrium constant expression. I will determine the solubilities of sparingly soluble ionic compounds.

EQUILIBRIUM: A STATE OF DYNAMIC BALANCE v I will recognize the characteristics of chemical equilibrium. v I will write equilibrium expressions for systems that are at equilibrium. v I will calculate equilibrium constants from concentration data.

COMPLETION v Reaction goes to completion • When a reaction results in almost complete conversion of reactants to products v Rarely happens

REVERSIBLE REACTION v Most reactions • Do NOT go to completion • Appear to stop • Are reversible v Reversible reaction • • One that can occur in both the forward and the reverse directions Denoted with a double arrow to show that both reactions occur Forward reaction = reactants on left Reverse reaction = reactants on right

REVERSIBLE REACTIONS
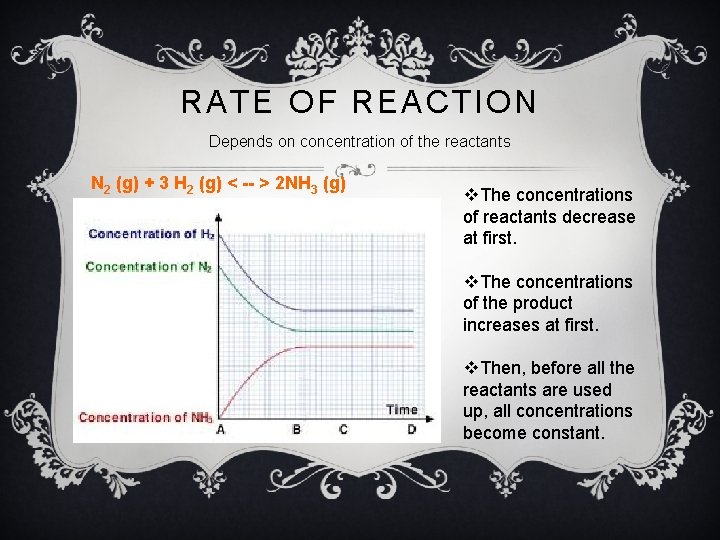
RATE OF REACTION Depends on concentration of the reactants N 2 (g) + 3 H 2 (g) < -- > 2 NH 3 (g) v. The concentrations of reactants decrease at first. v. The concentrations of the product increases at first. v. Then, before all the reactants are used up, all concentrations become constant.

CHEMICAL EQUILIBRIUM v A state in which the forward and reverse reactions balance each other because they take place at equal rates • Rate forward reaction = Rate reverse reaction • Concentrations of reactants and products are constant v HOWEVER! • The amounts or concentrations of reactants and products • Are NOT usually equal • MAY even differ by a factor of a million or more!

EQUILIBRIUM EXPRESSIONS AND CONSTANTS v Majority of reactions reach equilibrium with varying amounts of reactants unconsumed • NOT all our predicted moles of product gets produced v Law of Chemical Equilibrium • At a given temperature, a chemical system may reach a state in which a particular ratio of a reactant and product concentrations has a constant value • a. A + b. B <--> c. C + d. D

EQUILIBRIUM CONSTANT v The numerical value of the ratio of product concentrations to reactant concentrations v Constant only at a SPECIFIC TEMPERATURE v Products on top, reactants on bottom v Keq > 1: MORE products than reactants at equilibrium v Keq < 1: LESS products than reactants at equilibrium v Keq = [C]c[D]d [A]a[B]b

HOMOGENEOUS EQUILIBRUM v All the products and reactants are in the same physical state v Must use ALL CONCENTRATIONS for Keq

HETEROGENEOUS EQUILIBRIUM v Reactants and products of a reaction are present in more than one physical state v Do NOT count concentrations of solids or liquids when calculating Keq • Can be OMITTED from the Keq expressions v If a solid or liquid state of a substance is present in addition to the gas state…. LABEL the gas concentration in your expression to distinguish between the two

FACTORS AFFECTING CHEMICAL EQUILIBRIUM v I will describe how various factors affect chemical equilibrium. v I will explain how Le Chatelier’s Principle applies to equilibrium systems.

LE CHATELIER’S PRINCIPLE v Apply stress to a system at equilibrium v System will shift in the direction that relieves the stress v Stress • Any kind of change in a system at equilibrium that UPSETS equilibrium • Types: • Change in concentration • Change in volume (pressure) • Change in temperature
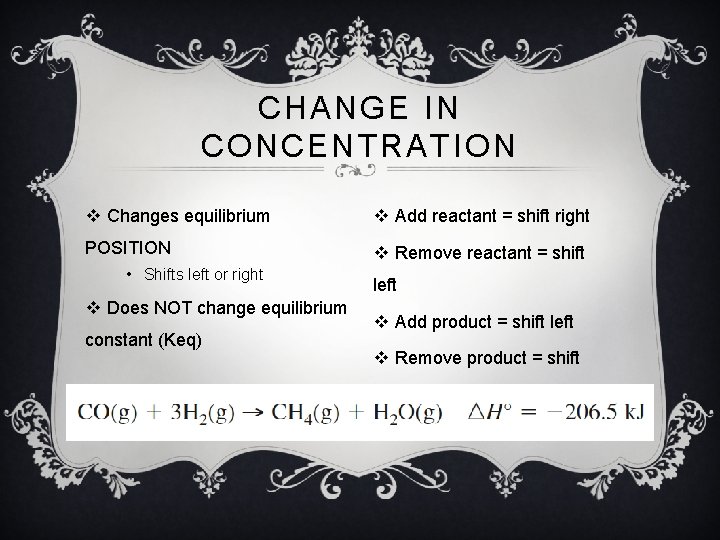
CHANGE IN CONCENTRATION v Changes equilibrium v Add reactant = shift right POSITION v Remove reactant = shift • Shifts left or right v Does NOT change equilibrium constant (Keq) left v Add product = shift left v Remove product = shift right

CHANGE IN VOLUME (PRESSURE) v Changes equilibrium POSITION • Shifts left or right • ONLY if # moles of gaseous reactants is DIFFERENT than # moles gaseous products v Does NOT change equilibrium constant (Keq) v Volume Pressure
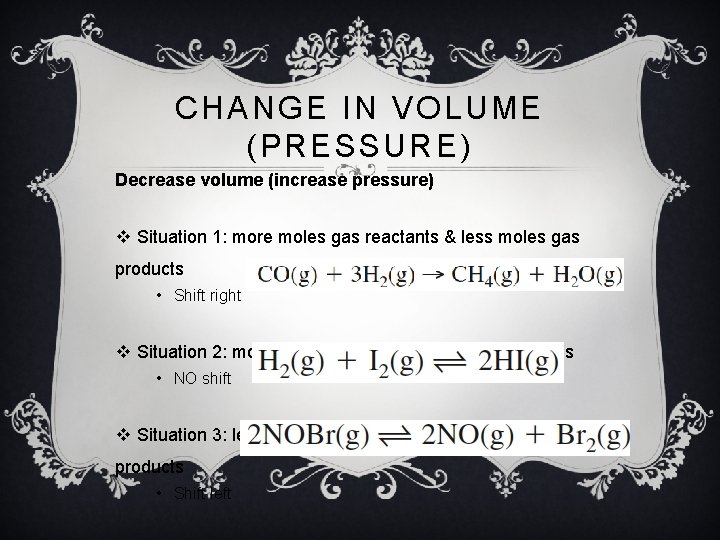
CHANGE IN VOLUME (PRESSURE) Decrease volume (increase pressure) v Situation 1: more moles gas reactants & less moles gas products • Shift right v Situation 2: moles gas reactants = moles gas products • NO shift v Situation 3: less moles gas reactants & more moles gas products • Shift left

CHANGE IN TEMPERATURE v Changes equilibrium POSITION • Shifts left or right v CHANGES equilibrium constant (Keq) • Large Keq = more product in equilibrium mixture • Small Keq = less product in equilibrium mixture
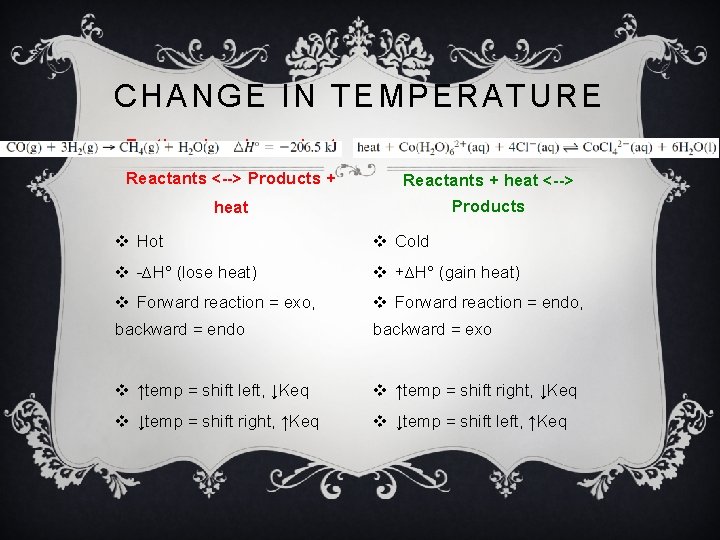
CHANGE IN TEMPERATURE Exothermic- releases heat Endothermic- absorbs heat Reactants <--> Products + Reactants + heat <--> heat Products v Hot v Cold v -∆H° (lose heat) v +∆H° (gain heat) v Forward reaction = exo, v Forward reaction = endo, backward = endo backward = exo v ↑temp = shift left, ↓Keq v ↑temp = shift right, ↓Keq v ↓temp = shift right, ↑Keq v ↓temp = shift left, ↑Keq


CATALYSTS v Speeds up a reaction v Speeds it up EQUALLY in BOTH directions (Right & Left) v Helps a reaction reach equilibrium quickly v But NO CHANGE in the AMOUNT of PRODUCT formed

USING EQUILIBRIUM CONSTANTS v I will determine equilibrium concentrations of reactants and products. v I will calculate the solubility of a compound from its solubility product constant. v I will explain the common ion effect.

- Slides: 22