Chemical Equilibrium Chapter 17 Equilibrium vs Kinetics speed

Chemical Equilibrium Chapter 17

Equilibrium vs. Kinetics: speed of a reaction or process how fast? Equilibrium: extent of reaction or process how much?
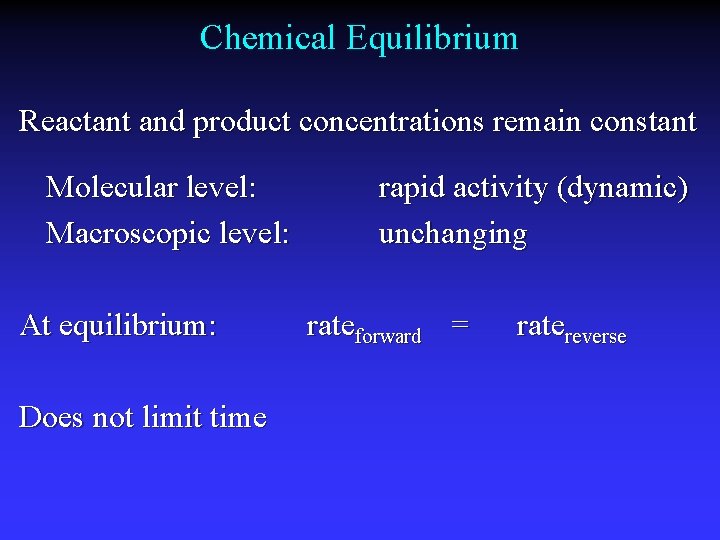
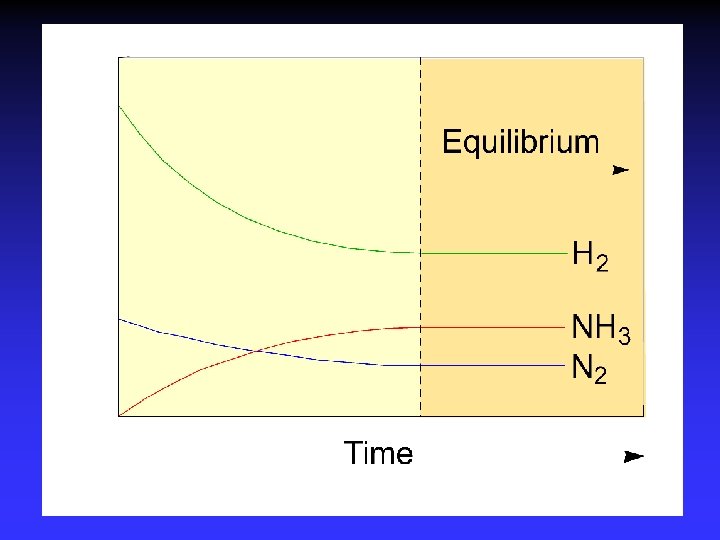
Chemical Equilibrium Reactant and product concentrations remain constant Molecular level: Macroscopic level: At equilibrium: Does not limit time rapid activity (dynamic) unchanging rateforward = ratereverse
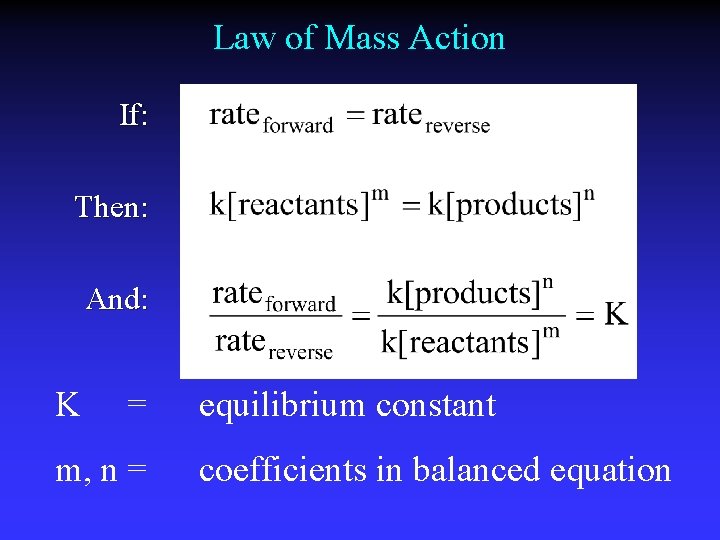
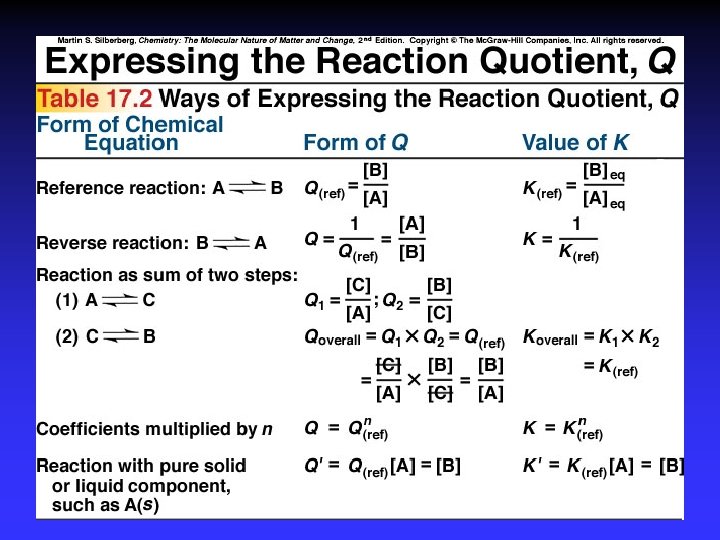
Law of Mass Action If: Then: And: K = m, n = equilibrium constant coefficients in balanced equation
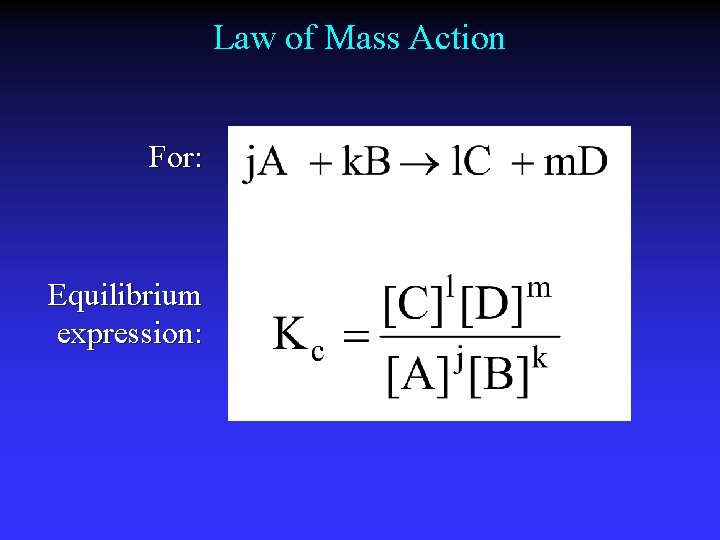
Law of Mass Action For: Equilibrium expression:
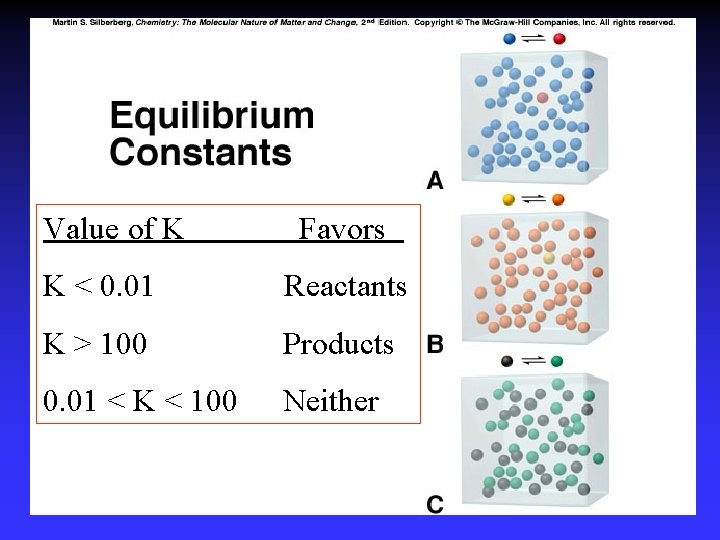
Value of K Favors K < 0. 01 Reactants K > 100 Products 0. 01 < K < 100 Neither
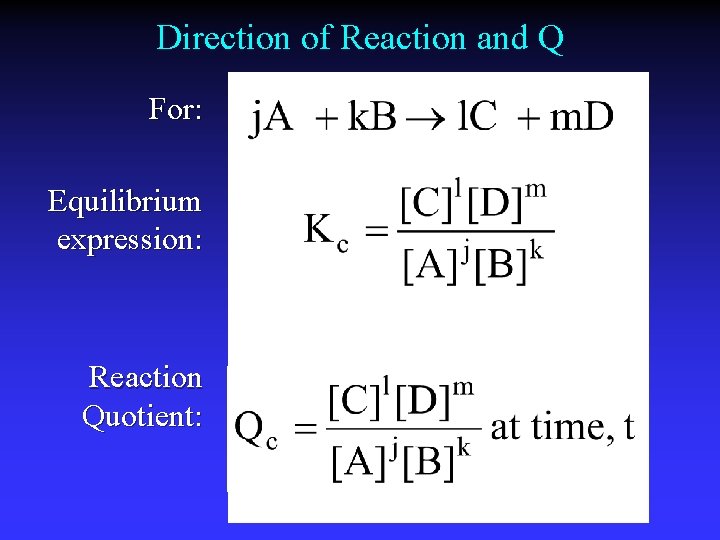
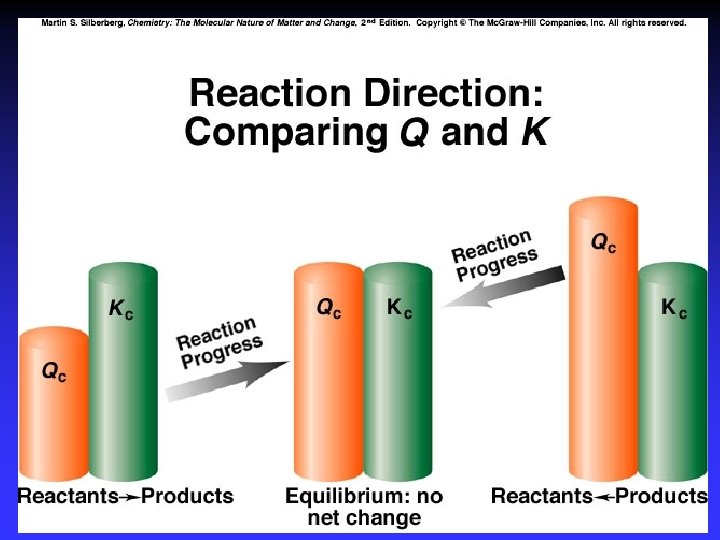
Direction of Reaction and Q For: Equilibrium expression: Reaction Quotient:
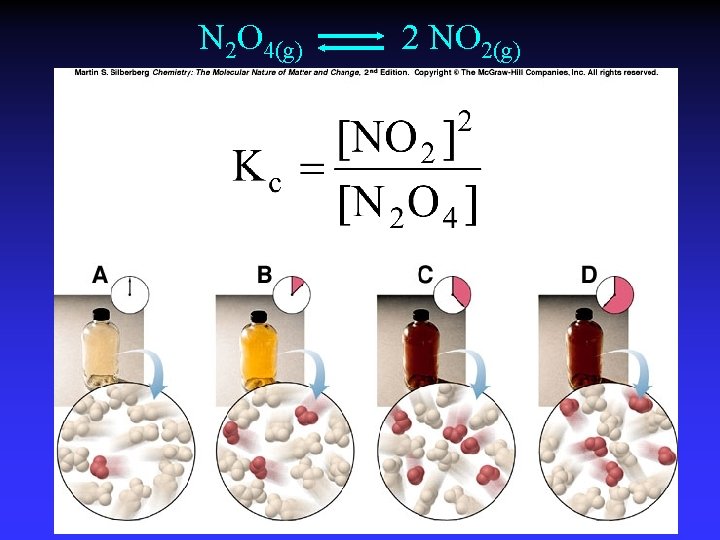
N 2 O 4(g) 2 NO 2(g)
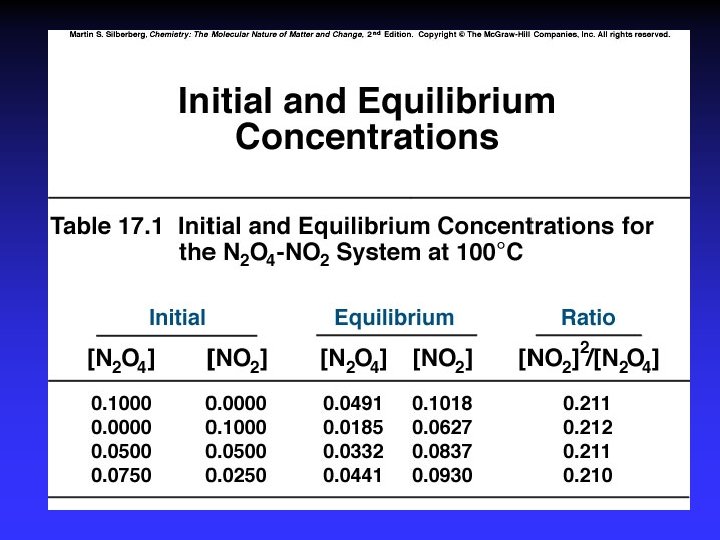
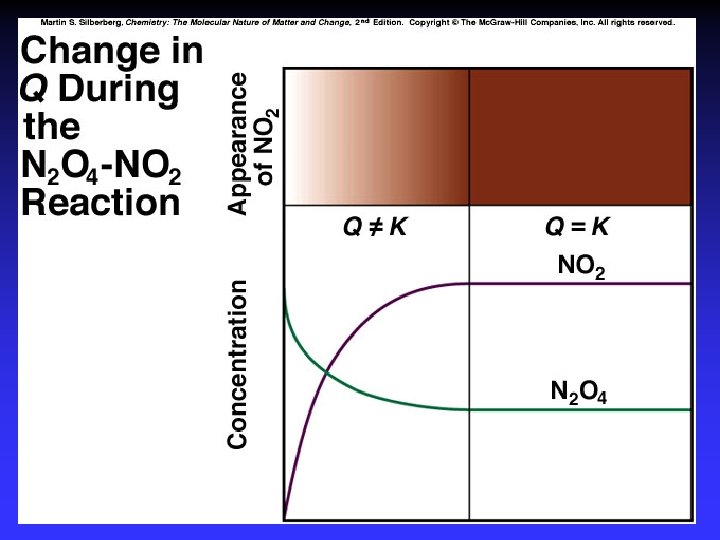

Practice 1. Writing expression for K 2. Q vs. K and reaction direction 3. K for a multistep process 4. K for reaction “multiples”

Write K The decomposition of dinitrogen pentoxide: N 2 O 5(g) NO 2(g) + O 2(g) The combustion of propane gas: C 3 H 8(g) + O 2(g) CO 2(g) + H 2 O(g O( )
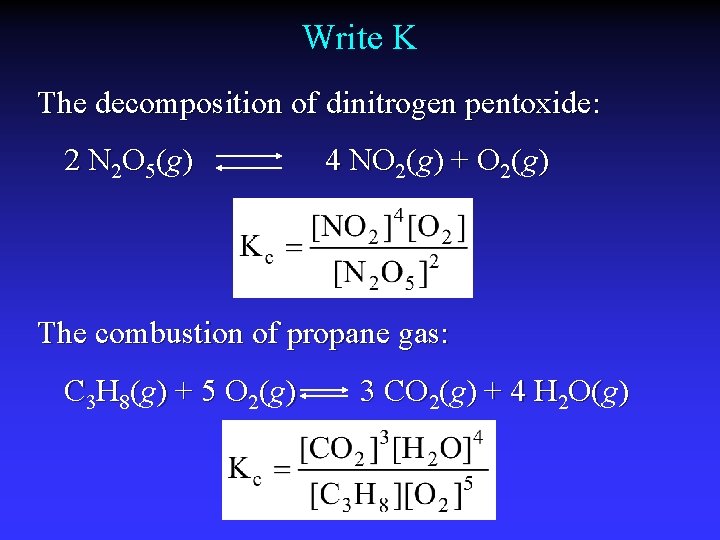
Write K The decomposition of dinitrogen pentoxide: 2 N 2 O 5(g) 4 NO 2(g) + O 2(g) The combustion of propane gas: C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(g O( )
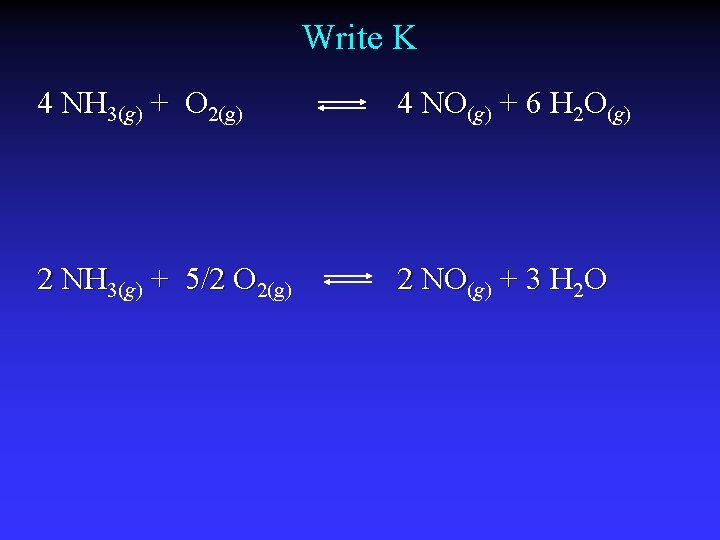
Write K 4 NH 3(g) + O 2(g) 4 NO(g) + 6 H 2 O(g) 2 NH 3(g) + 5/2 O 2(g) 2 NO(g) + 3 H 2 O

K vs. Q For the reaction: N 2 O 4(g) Kc = 0. 21 at 1000 C. 2 NO 2(g) At a point during the reaction, [N 2 O 4] = 0. 12 M and [NO 2] = 0. 55 M. (a) Find Q. Is the reaction at equilibrium? (b) If not, in which direction is it progressing?

K vs. Q N 2 O 4(g) 2 NO 2(g) Kc = 0. 21 at 1000 C. At a point, [N 2 O 4] = 0. 12 M and [NO 2] = 0. 55 M. (a) Find Q. Is the reaction at equilibrium? (b) If not, in which direction is it progressing?
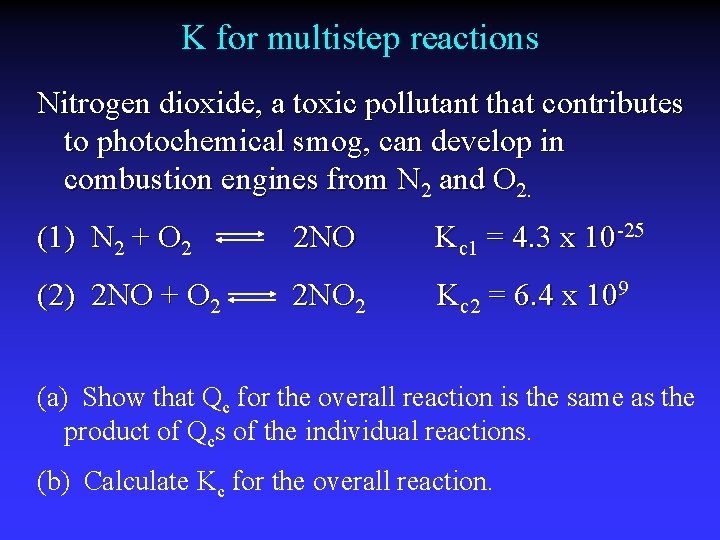
K for multistep reactions Nitrogen dioxide, a toxic pollutant that contributes to photochemical smog, can develop in combustion engines from N 2 and O 2. (1) N 2 + O 2 2 NO Kc 1 = 4. 3 x 10 -25 (2) 2 NO + O 2 2 NO 2 Kc 2 = 6. 4 x 109 (a) Show that Qc for the overall reaction is the same as the product of Qcs of the individual reactions. (b) Calculate Kc for the overall reaction.
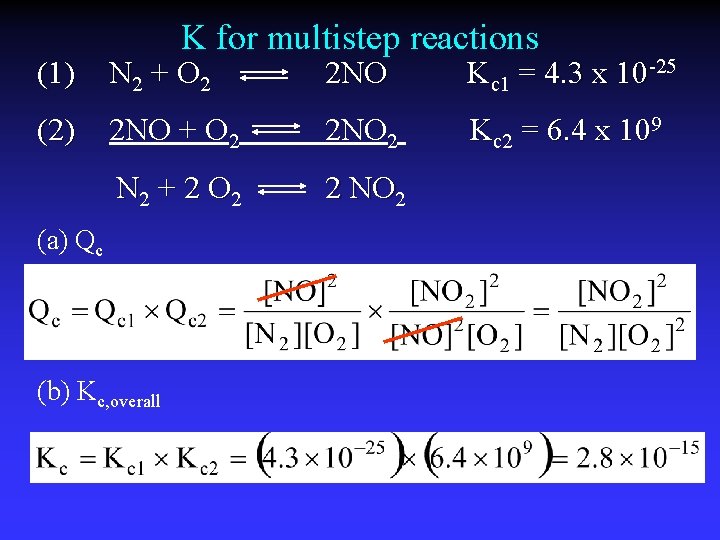
K for multistep reactions (1) N 2 + O 2 2 NO Kc 1 = 4. 3 x 10 -25 (2) 2 NO + O 2 2 NO 2 Kc 2 = 6. 4 x 109 N 2 + 2 O 2 2 NO 2 (a) Qc (b) Kc, overall
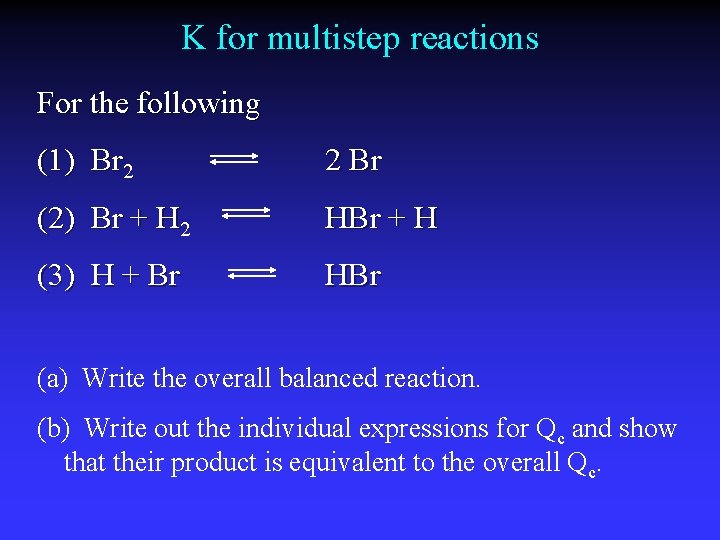
K for multistep reactions For the following (1) Br 2 2 Br (2) Br + H 2 HBr + H (3) H + Br HBr (a) Write the overall balanced reaction. (b) Write out the individual expressions for Qc and show that their product is equivalent to the overall Qc.

Multiples of K For the ammonia reaction: N 2(g) + 3 H 2(g) Kc is 2. 4 x 10 -3 at 1000 K. Find K for the following: (a) 1/3 N 2+ H 2 (b) NH 3 2/3 NH 3 1/2 N 2 + 3/2 H 2 2 NH 3(g)

Multiples of K N 2(g) + 3 H 2(g) 2 NH 3(g), Kc = 2. 4 x 10 -3 (a) 1/3 N 2+ H 2 2/3 NH 3 (b) NH 3 1/2 N 2 + 3/2 H 2

Multiples N 2(g) + O 2(g) 2 NO(g) Kc = 1 x 10 -30 Write the expression for Q and determine its value for ½ N 2(g) + ½ O 2(g) 2 NO(g) H 2(g) + Cl 2(g) 2 HCl(g) Kc = 7. 6 x 108 Write the expression for Q and determine its value for 2/3 HCl(g) 1/3 H 2(g) + 1/3 Cl 2(g)
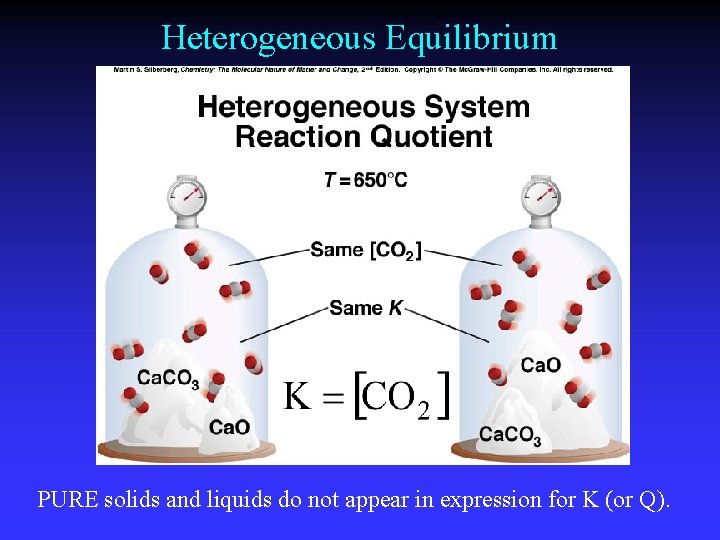
Heterogeneous Equilibrium PURE solids and liquids do not appear in expression for K (or Q).
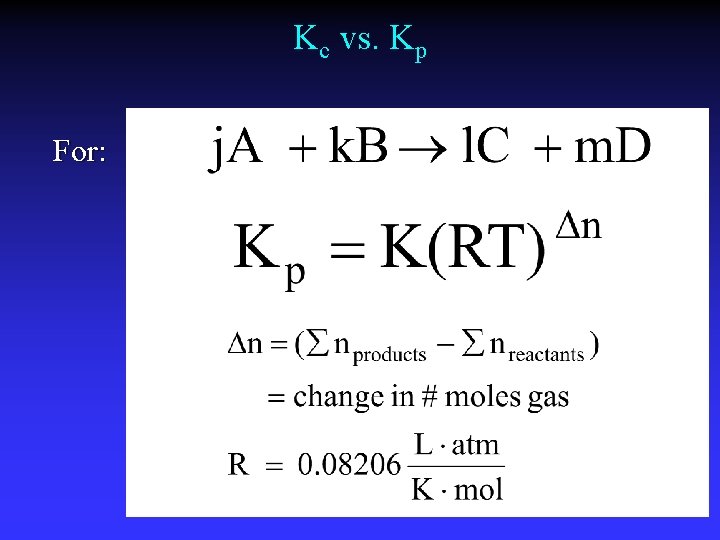
Kc vs. Kp For:

Kp and Kc For the ammonia reaction, N 2(g) + 3 H 2(g) 2 NH 3(g), Kc = 2. 4 x 10 -3 Find Kp at 1000 K.

Kp and Kc N 2(g) + 3 H 2(g) 2 NH 3(g), Kc = 2. 4 x 10 -3 Find Kp at 1000 K.

Kp and Kc For the following reaction, PCl 3(g) + Cl 2(g) PCl 5(g), Kc = 1. 67 at 500 K Find Kp at 500 K.
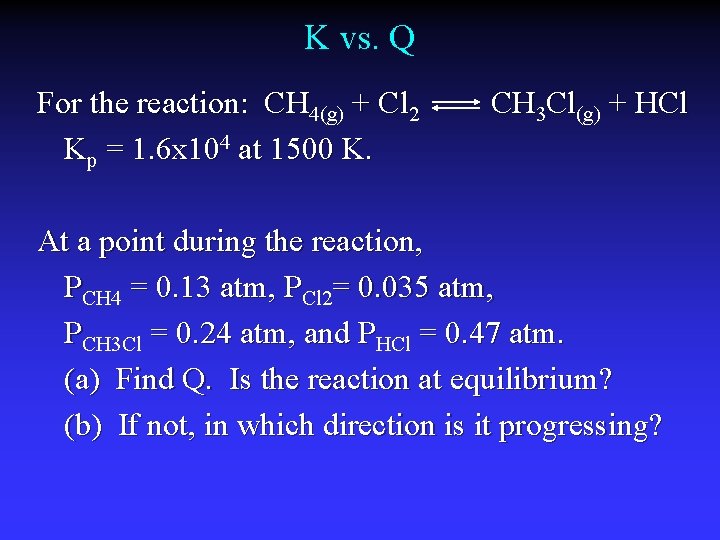
K vs. Q For the reaction: CH 4(g) + Cl 2 Kp = 1. 6 x 104 at 1500 K. CH 3 Cl(g) + HCl At a point during the reaction, PCH 4 = 0. 13 atm, PCl 2= 0. 035 atm, PCH 3 Cl = 0. 24 atm, and PHCl = 0. 47 atm. (a) Find Q. Is the reaction at equilibrium? (b) If not, in which direction is it progressing?

Problems 1. Given equilibrium concentrations or pressures, find K or Q. 2. Given K and initial conditions (conc’s or P’s), find equilibrium quantities (conc’s or P’s).


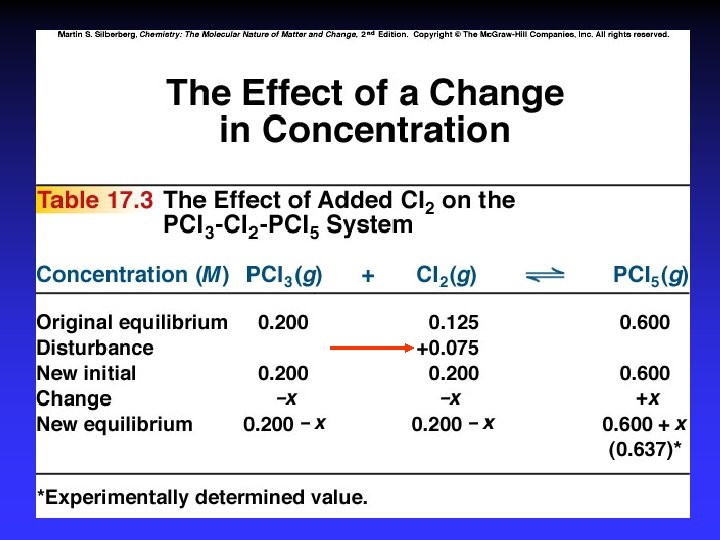
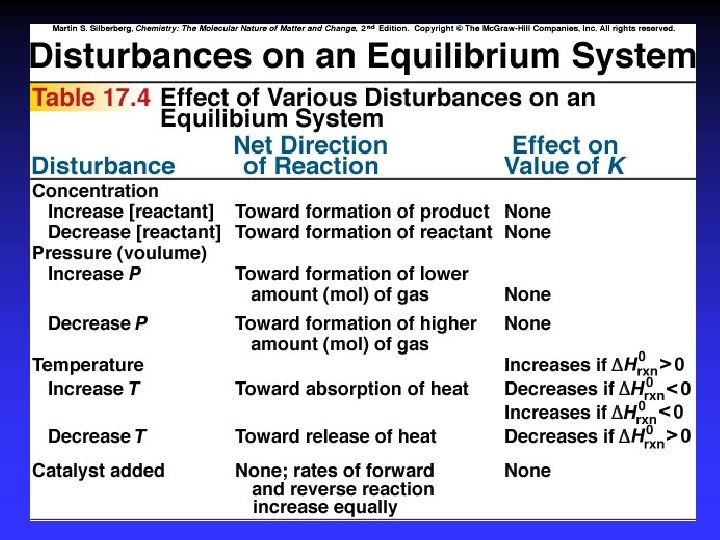
Le Châtelier’s Principle . . . if a change is imposed on a system at equilibrium, the position of the equilibrium will shift in a direction that tends to reduce that change.

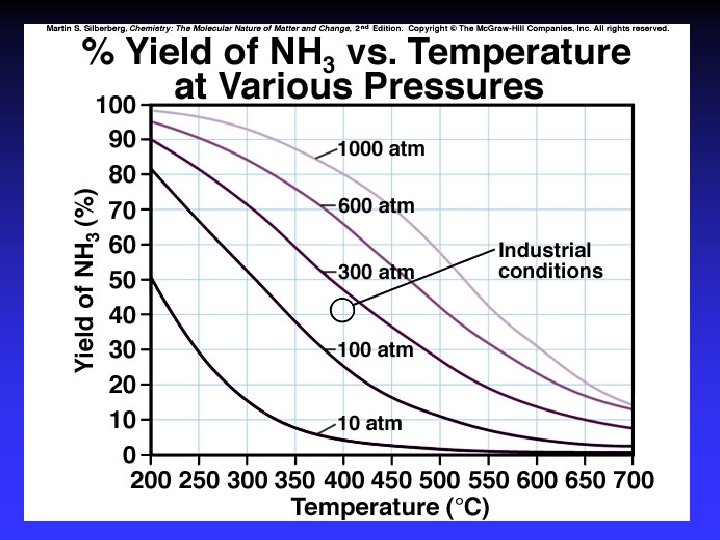
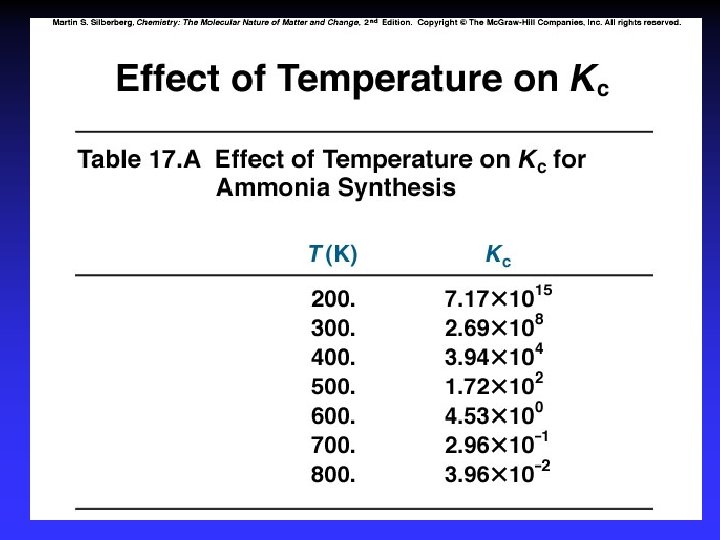
Le Châtelier’s Principle 1. 2. 3. 4. 5. Concentration Temperature Pressure Volume Catalysts*
- Slides: 40