Chemical Equilibrium Chapter 17 Chemical Equilibrium Chemical Equilibrium

Chemical Equilibrium Chapter 17

Chemical Equilibrium • Chemical Equilibrium is a state of dynamic balance where the rate of the forward reaction is equal to the rate of the reverse reaction.

Le Chatelier’s Principle • When a system in equilibrium is subjected to a stress, the system readjusts to relieve the effect of the stress. • Stresses include Pressure, Temperature, Concentration of one or more reactants. • Leaving Cert 2008 Q 7, 2007 Q 10 a, 2006 Q 11 b, 2005 Q 9 2004 Q 9(C) 2003 Q 11 2002 Q 10(C)
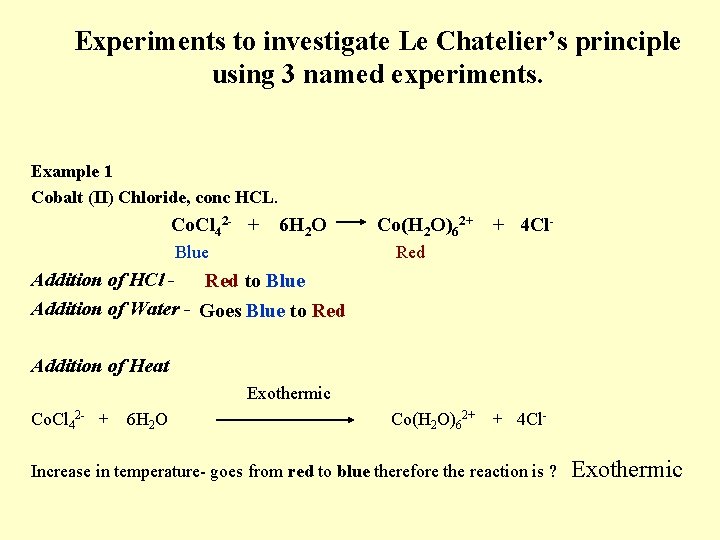
Experiments to investigate Le Chatelier’s principle using 3 named experiments. Example 1 Cobalt (II) Chloride, conc HCL. Co. Cl 42 - + 6 H 2 O Blue Co(H 2 O)62+ + 4 Cl- Red Addition of HCl Red to Blue Addition of Water - Goes Blue to Red Addition of Heat Exothermic Co. Cl 42 - + 6 H 2 O Co(H 2 O)62+ + 4 Cl- Increase in temperature- goes from red to blue therefore the reaction is ? Exothermic
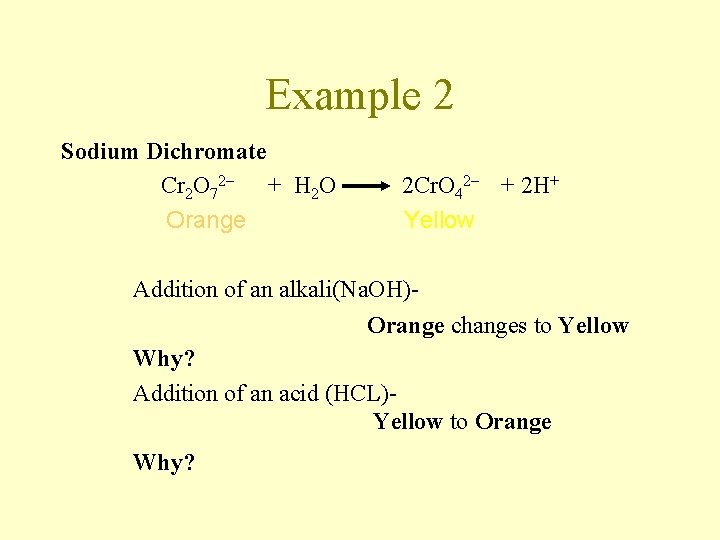
Example 2 Sodium Dichromate Cr 2 O 72– + H 2 O Orange 2 Cr. O 42– + 2 H+ Yellow Addition of an alkali(Na. OH)Orange changes to Yellow Why? Addition of an acid (HCL)Yellow to Orange Why?
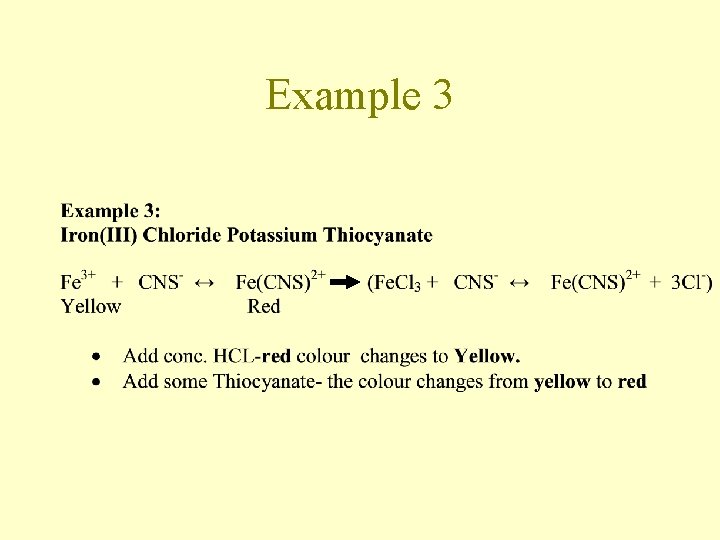
Example 3
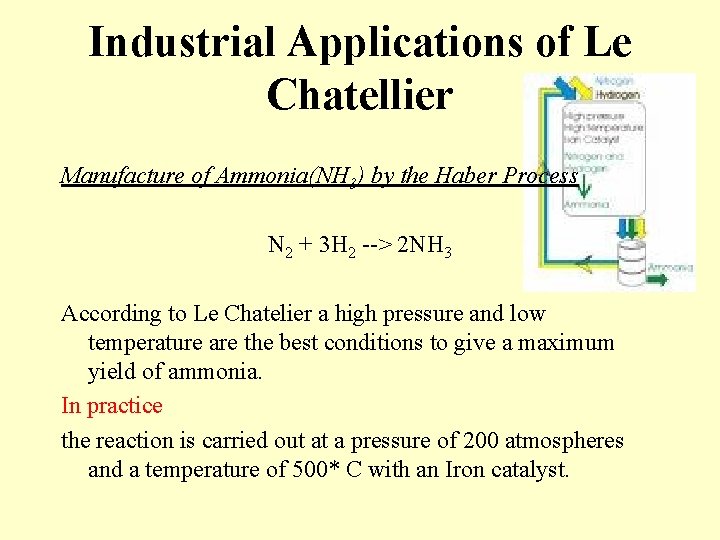
Industrial Applications of Le Chatellier Manufacture of Ammonia(NH 3) by the Haber Process N 2 + 3 H 2 --> 2 NH 3 According to Le Chatelier a high pressure and low temperature are the best conditions to give a maximum yield of ammonia. In practice the reaction is carried out at a pressure of 200 atmospheres and a temperature of 500* C with an Iron catalyst.
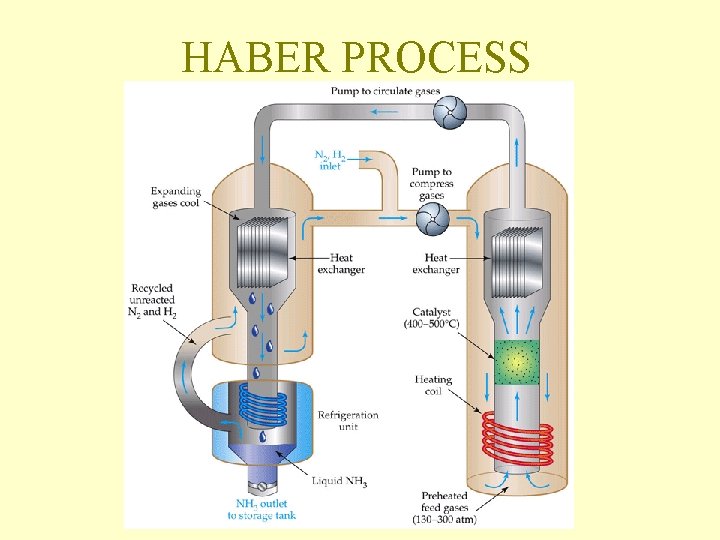
HABER PROCESS
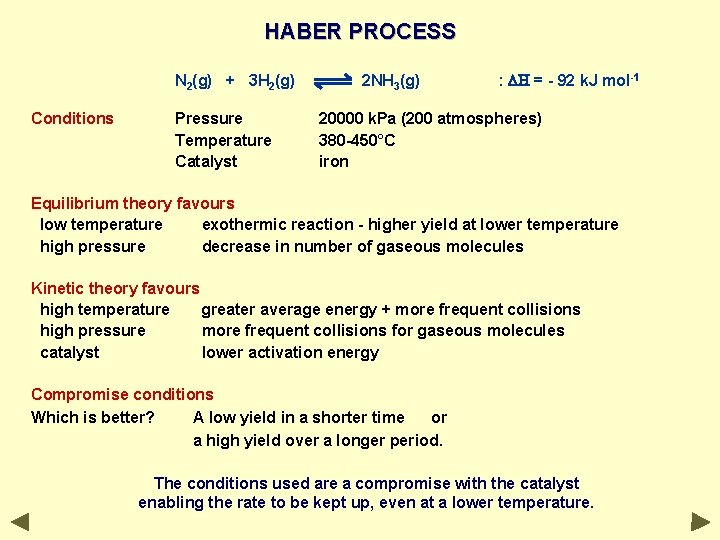
HABER PROCESS N 2(g) + 3 H 2(g) Conditions Pressure Temperature Catalyst 2 NH 3(g) : DH = - 92 k. J mol-1 20000 k. Pa (200 atmospheres) 380 -450°C iron Equilibrium theory favours low temperature exothermic reaction - higher yield at lower temperature high pressure decrease in number of gaseous molecules Kinetic theory favours high temperature greater average energy + more frequent collisions high pressure more frequent collisions for gaseous molecules catalyst lower activation energy Compromise conditions Which is better? A low yield in a shorter time or a high yield over a longer period. The conditions used are a compromise with the catalyst enabling the rate to be kept up, even at a lower temperature.
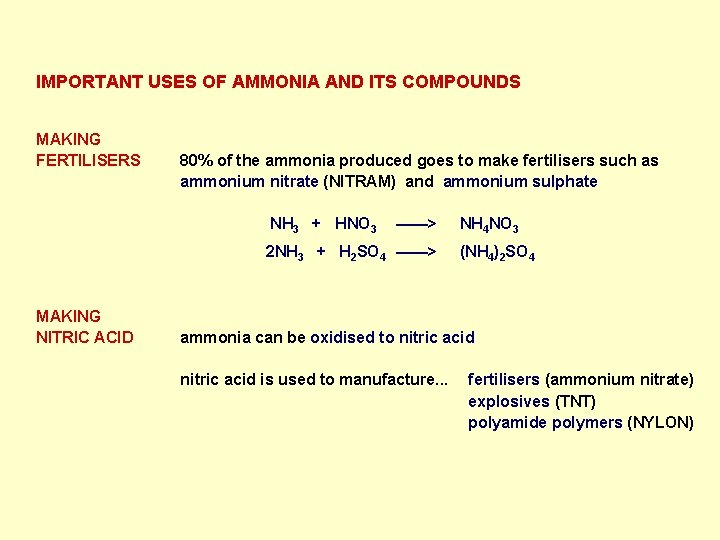
IMPORTANT USES OF AMMONIA AND ITS COMPOUNDS MAKING FERTILISERS 80% of the ammonia produced goes to make fertilisers such as ammonium nitrate (NITRAM) and ammonium sulphate NH 3 + HNO 3 ——> 2 NH 3 + H 2 SO 4 ——> MAKING NITRIC ACID NH 4 NO 3 (NH 4)2 SO 4 ammonia can be oxidised to nitric acid is used to manufacture. . . fertilisers (ammonium nitrate) explosives (TNT) polyamide polymers (NYLON)
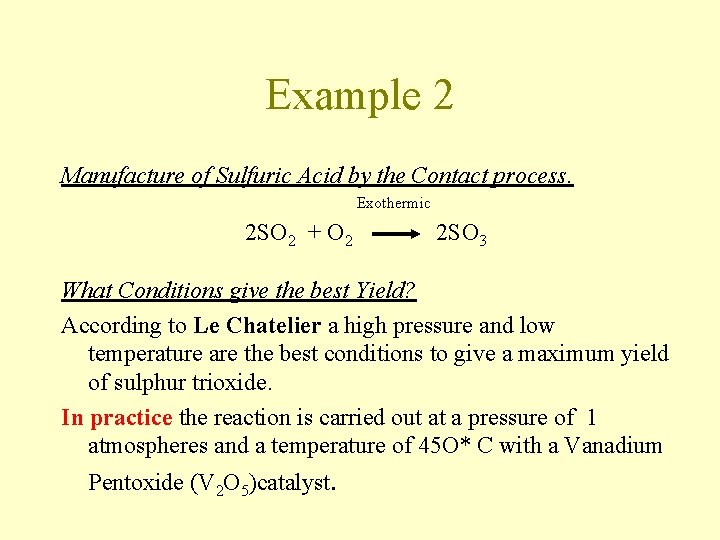
Example 2 Manufacture of Sulfuric Acid by the Contact process. Exothermic 2 SO 2 + O 2 2 SO 3 What Conditions give the best Yield? According to Le Chatelier a high pressure and low temperature are the best conditions to give a maximum yield of sulphur trioxide. In practice the reaction is carried out at a pressure of 1 atmospheres and a temperature of 45 O* C with a Vanadium Pentoxide (V 2 O 5)catalyst.
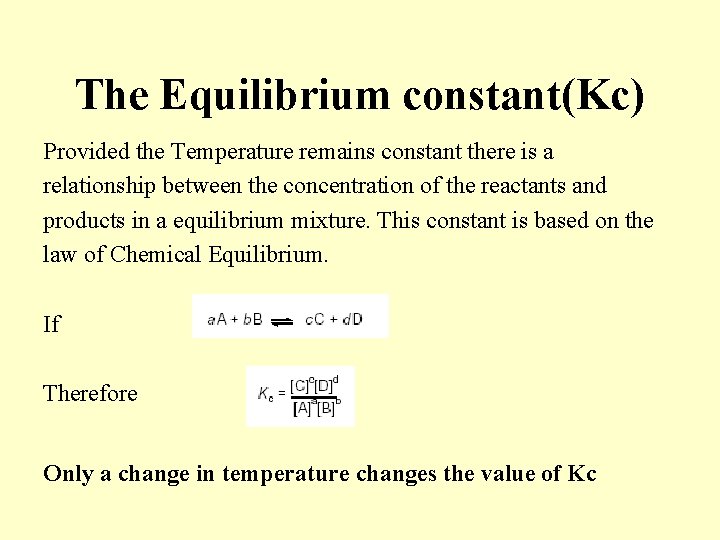
The Equilibrium constant(Kc) Provided the Temperature remains constant there is a relationship between the concentration of the reactants and products in a equilibrium mixture. This constant is based on the law of Chemical Equilibrium. If Therefore Only a change in temperature changes the value of Kc
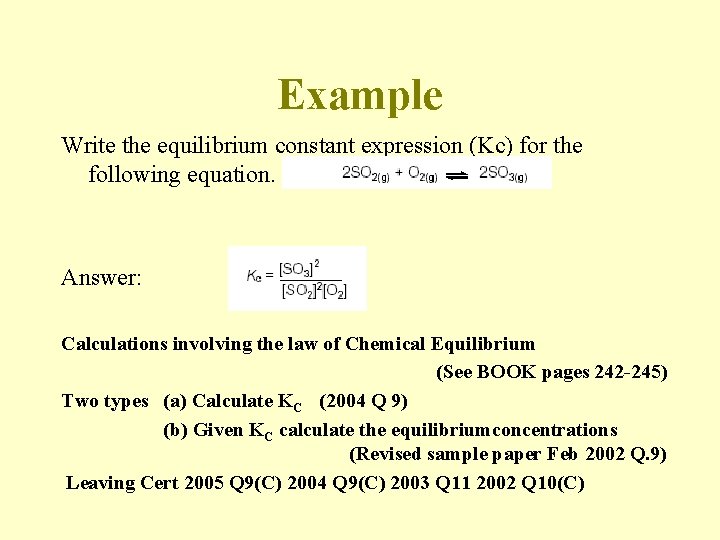
Example Write the equilibrium constant expression (Kc) for the following equation. Answer: Calculations involving the law of Chemical Equilibrium (See BOOK pages 242 -245) Two types (a) Calculate KC (2004 Q 9) (b) Given KC calculate the equilibriumconcentrations (Revised sample paper Feb 2002 Q. 9) Leaving Cert 2005 Q 9(C) 2004 Q 9(C) 2003 Q 11 2002 Q 10(C)

Question 7 2010 paper Find KC

Given KC find the equilibrium concentrations. 2003 Q 11(a)
- Slides: 15