Chemical Equilibrium Chapter 16 Hein and Arena Version

Chemical Equilibrium Chapter 16 Hein and Arena Version 2. 0 12 th Edition Eugene Passer Chemistry Department Bronx Community 1 College © John Wiley and Sons, Inc

Chapter Outline 16. 1 Reversible Reactions 16. 2 Rates of Reaction 16. 3 Chemical Equilibrium 16. 4 Le Chatelier’s Principle 16. 8 Effect of Catalysts on Equilibrium 16. 9 Equilibrium Constants 16. 10 Ion Product Constant for Water 16. 5 Effect of Concentration on Equilibrium 16. 11 Ionization Constants 16. 6 Effect of Volume on Equilibrium 16. 13 Acid-Base Properties of Salts 16. 7 Effect of Temperature on Equilibrium 16. 14 Buffer Solutions: The Control of p. H 16. 12 Solubility Product Constant 2

16. 1 Reversible Reactions 3

reversible reaction: A chemical reaction in which the products formed react to produce the original reactants. 4

The reaction between NO 2 and N 2 O 4 is reversible. cooling N 2 O 4 is formed 2 NO 2(g) → N 2 O 4 (g) N 2 O 4 decomposes when heated, forming NO 2 heating N 2 O 4 (g) → 2 NO 2 (g) 5

reaction to the right 2 NO 2(g) → N 2 O 4 (g) → reaction to the left 6

16. 2 Rates of Reaction 7

chemical kinetics The study of reaction rates and reaction mechanisms. 8

• The rate of a reaction is variable. It depends on: – concentrations of the reacting species – reaction temperature – presence or absence of catalysts – the nature of the reactants 9

The concentration of A and B decreases with time, lowering the rate of the forward reaction. The concentration of C and D increases with time, increasing the rate of the reverse reaction. Forward reaction A + B → C + D Reverse reaction C + D → A + B 16. 2 10

16. 3 Chemical Equilibrium 11

equilibrium: a the dynamic state which At equilibrium concentrations of the chemical equilibrium: the in state in two orthemore processes are products and thereactants are not which rate opposing of forward reaction taking the place time and at in thea changing. equals rateatofthe thesame reverse reaction same rate. change. chemical 12

A saturated salt solution is in equilibrium with solid salt crystals are dissolving Na+ and Clare crystallizing Na. Cl(s) → Na+(aq) + Cl-(aq) → At equilibrium, the rate of salt dissolution equals the rate of salt crystallization. 13

16. 4 Le Chatelier’s Principle 14

In 1888, the French chemist Henri Le. Chatelier This generalization, known as set forth a far-reaching generalization on the Le. Chatelier’s Principle, states behavior of equilibrium systems. If a stress or strain is applied to a system in equilibrium, the system will respond in such a way as to relieve that stress and restore equilibrium under a new set of conditions. 15

16. 5 Effect of Concentration on Equilibrium 16
• For most reactions, the rate of reaction increases as reactant concentrations increase. • The manner in which the rate of reaction changes with concentration must be determined experimentally. 17

An equilibrium is disturbed when the concentration of one or more of its components is changed. As a result, the concentration of all species will change and a new equilibrium mixture will be established. 18
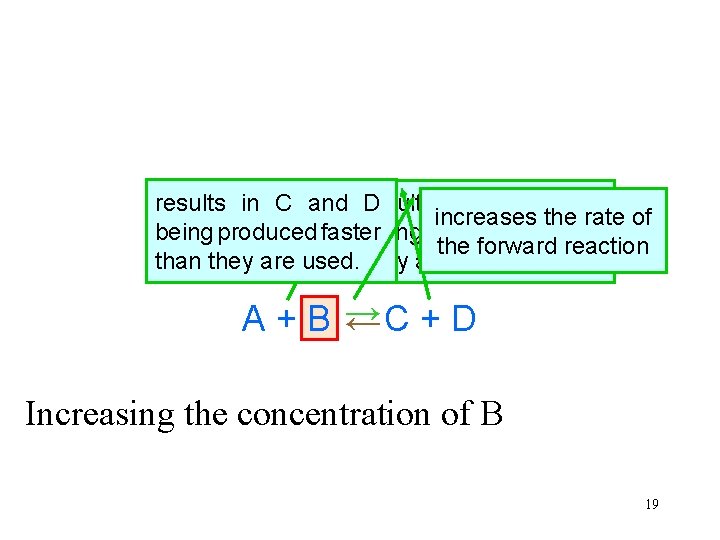
The system is at equilibrium results in C and D results in A and B increases the rate of being produced faster being used faster than the forward reaction than they are used. they are produced. A + B →C + D → Increasing the concentration of B 19

The system is again at equilibrium In the new equilibrium concentration of A has decreased concentrations of B, C and D have increased A+B→C+D → After enough time has passed, the rates of the forward and reverse reactions become equal. 20

Percent Yield 21

At. The equilibrium forwardthe reaction rate ofis. HI 79% formation complete equals at the equilibrium. rate of HI decomposition. H 2 + I 2 combine to form HI HI decomposes to form H 2 + I 2 700 K H 2(g) + I 2(g) → 2 HI(aq) → 0. 21 10 mol 0. 21 01 mol 1. 58 20 mol Final Concentrations in the Equilibrium Initial Concentrations Absence of Equilibrium 22

Comparison of Equilibria Original Equilibrium New Equilibrium 1. 00 mol H 2 + 1. 00 mol I 2 1. 00 mol H 2 + 1. 20 mol I 2 Yield: 79% HI Yield: 85% HI Equilibrium mixture contains 1. 58 mol HI 1. 70 mol HI 0. 21 mol H 2 0. 15 mol H 2 0. 21 mol I 2 0. 35 mol I 2 23
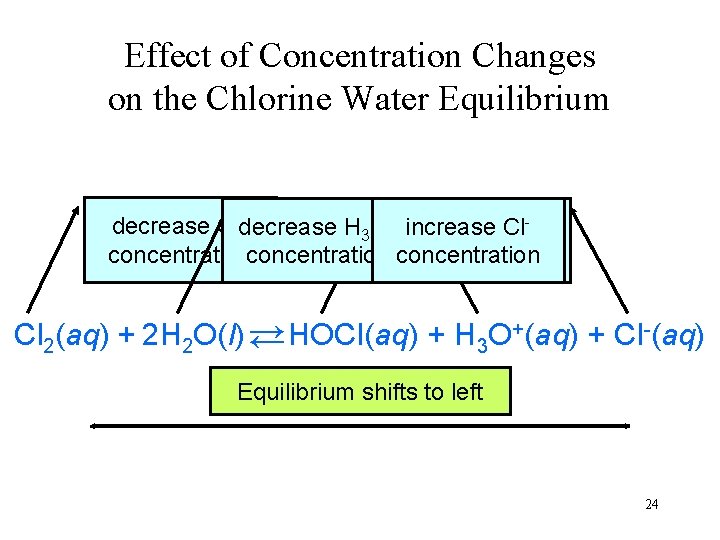
Effect of Concentration Changes on the Chlorine Water Equilibrium +increase decrease Cldecrease increase H H O O increase HOCl Cl 2 32 concentrationconcentration Cl 2(aq) + 2 H 2 O(l) → HOCl(aq) + H 3 O+(aq) + Cl-(aq) → Equilibriumshiftsto toright left 24

Effect of C 2 H 3 O 2 Concentration Changes on p. H Add 0. 200 0. 100 mol Na. C 2 H 3 O 2(aq) → Na+(aq) + C 2 H 3 O 2(aq) Equilibrium shifts to left HC 2 H 3 O 2(aq) + H 2 O(l) → H 3 O+(aq) + C 2 H 3 O 2(aq) → 1 L 0. 100 M HC 2 H 3 O 2 Equilibrium p. H = 5. 05 2. 87 4. 74 25

16. 6 Effect of Volume on Equilibrium 26

• Changes in volume significantly affect the reaction rate only when one or more of the reactants or products is a gas and the reaction is run in a closed container. • The effect of decreasing the volume is to increase the concentrations of any gaseous reactants or products. 27

Decrease Volume increases CO 2 concentration Ca. CO 3(s) → Ca. O(s) + CO 2(g) → Equilibrium shifts to left 28

Increase Volume decreases CO 2 concentration Ca. CO 3(s) → Ca. O(s) + CO 2(g) → Equilibrium shifts to right 29

In a system composed entirely of gases, a decrease in the volume of the container will cause the reaction and the equilibrium to shift to the side that contains the smallest number of molecules. 30
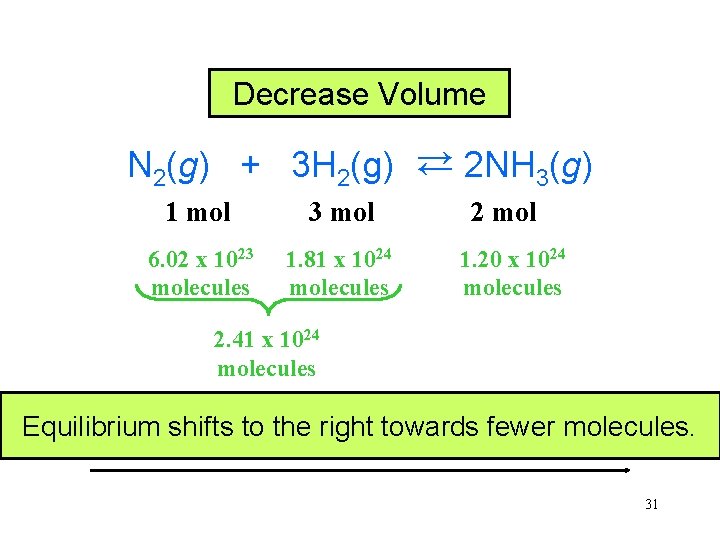
Decrease Volume N 2(g) + 3 H 2(g) → 2 NH 3(g) → 1 mol 3 mol 6. 02 x 1023 molecules 1. 81 x 1024 molecules 2 mol 1. 20 x 1024 molecules 2. 41 x 1024 molecules Equilibrium shifts to the right towards fewer molecules. 31

Decrease Volume N 2(g) + O 2(g) → 2 NO(g) → 1 mol 6. 02 x 1023 molecules 2 mol 1. 20 x 1024 molecules Equilibrium does not shift. The number of molecules is the same on both sides of the equation. 32

16. 7 Effect of Temperature on Equilibrium 33

Thea rate In reversible of the reaction, reaction that the rates absorbs of both heat When the temperature of a system is theincreased is forward and to athe greater reverse extent, reactions and the are raised, the rate of reaction increases. equilibrium to favorinthat reaction. increased byshifts an increase temperature. 34
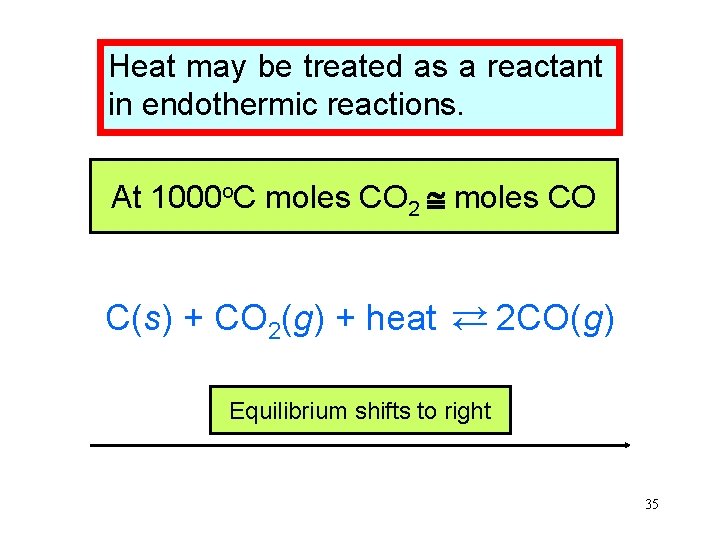
Heat may be treated as a reactant in endothermic reactions. o. C moles CO At room At 1000 temperature very moles COCO forms. 2 little C(s) + CO 2(g) + heat → 2 CO(g) → Equilibrium shifts to right 35

16. 8 Effect of Catalysts on Equilibrium 36
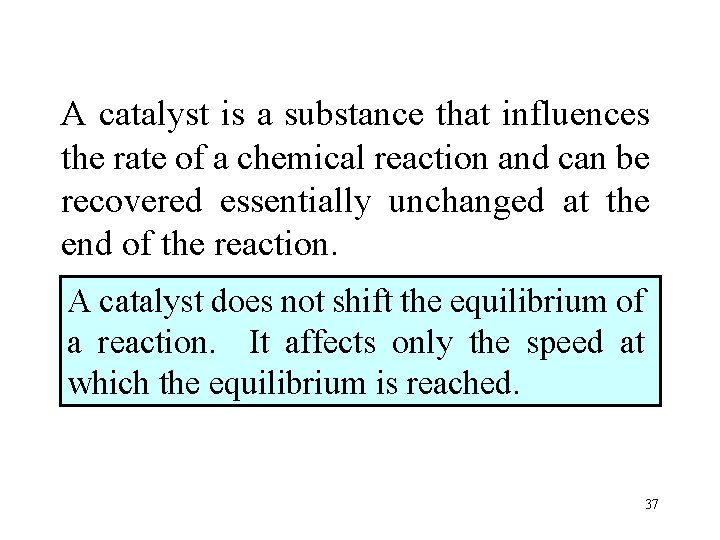
A catalyst is a substance that influences the rate of a chemical reaction and can be recovered essentially unchanged at the end of the reaction. A catalyst does not shift the equilibrium of a reaction. It affects only the speed at which the equilibrium is reached. 37
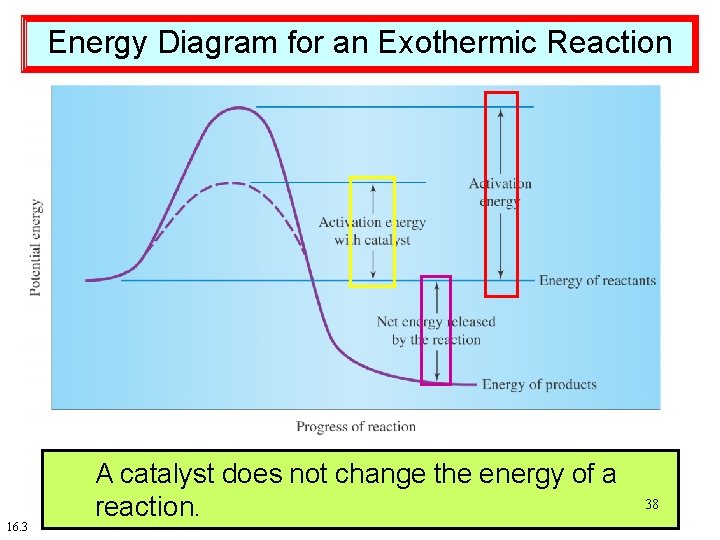
Energy Diagram for an Exothermic Reaction 16. 3 Activation A catalyst energy: speeds does not the up achange minimum reaction the energy byenergy lowering required of athe 38 for activation reaction. a reaction energy. to occur.
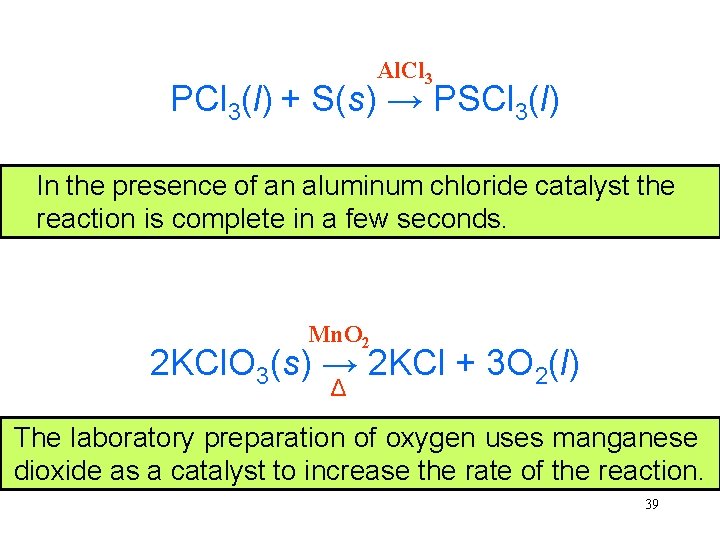
Al. Cl 3 PCl 3(l) + S(s) → PSCl 3(l) Very In thelittle presence thiophosphoryl of an aluminum chloride is formed catalyst in the absence reaction of is a complete catalyst in because a few seconds. the reaction is so slow. Mn. O 2 2 KCl. O 3(s) → 2 KCl + 3 O 2(l) Δ The laboratory preparation of oxygen uses manganese dioxide as a catalyst to increase the rate of the reaction. 39

16. 9 Equilibrium Constants 40

At equilibrium the rates of the forward and reverse reactions are equal, and the concentrations of the reactants and products are constant. 41

The equilibrium constant (Keq) is a value representing the unchanging concentrations of the reactants and the products in a chemical reaction at equilibrium. 42

For the general reaction a. A + b. B → c. C + d. D → at a given temperature 43
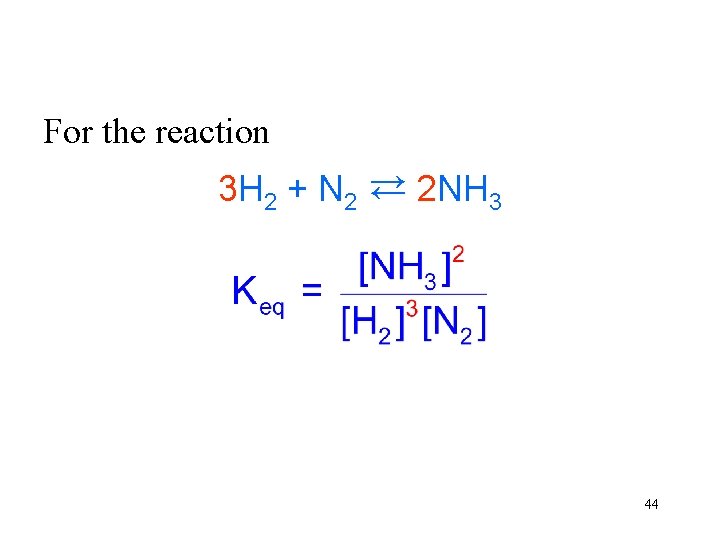
For the reaction 3 H 2 + N 2 → 2 NH 3 → 44
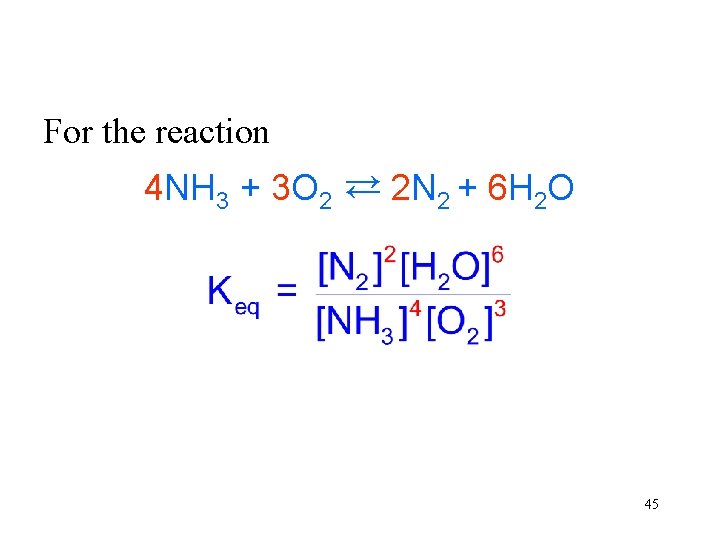
For the reaction 4 NH 3 + 3 O 2 → 2 N 2 + 6 H 2 O → 45
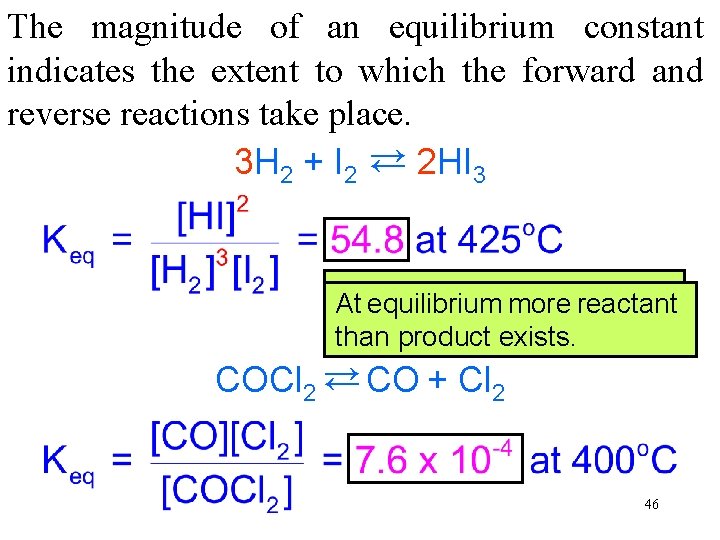
The magnitude of an equilibrium constant indicates the extent to which the forward and reverse reactions take place. 3 H 2 + I 2 → 2 HI 3 → At At equilibrium more product reactant than exists. than reactant product exists. COCl 2 → CO + Cl 2 → 46

When the molar concentrations of all species in an equilibrium reaction are known, the Keq can be calculated by substituting the concentrations into the equilibrium constant expression. 47
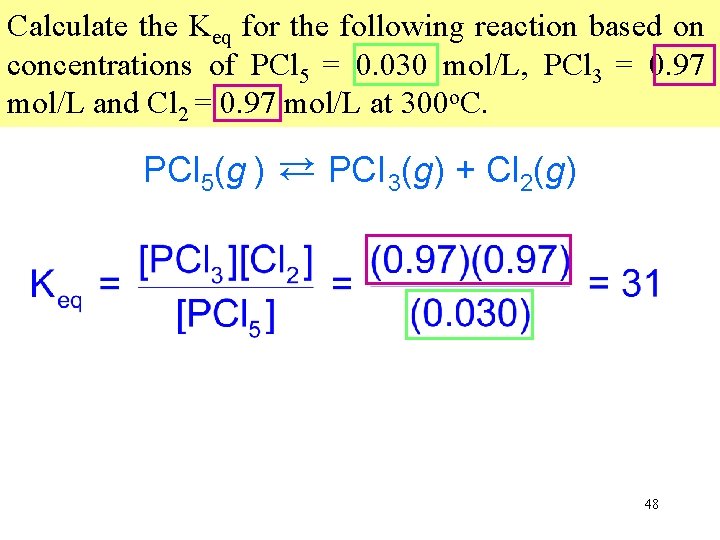
Calculate the Keq for the following reaction based on concentrations of PCl 5 = 0. 030 mol/L, PCl 3 = 0. 97 mol/L and Cl 2 = 0. 97 mol/L at 300 o. C. PCl 5(g ) → PCI 3(g) + Cl 2(g) → 48

16. 10 Ion Product Constant for Water 49
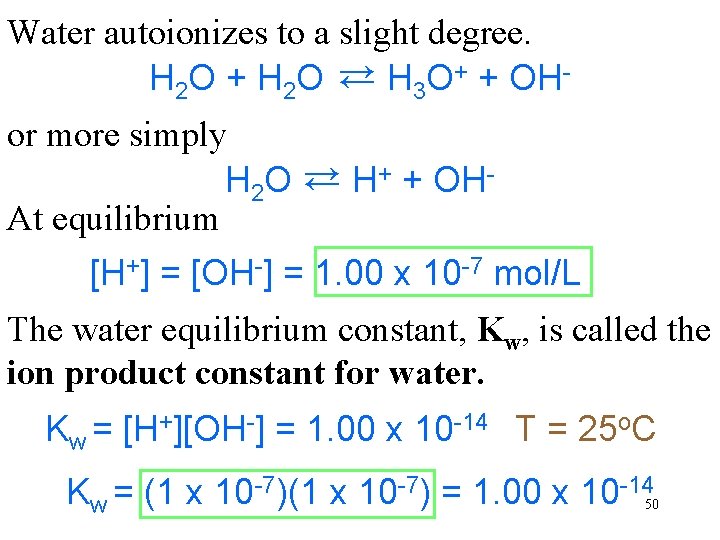
Water autoionizes to a slight degree. H 2 O + H 2 O → H 3 O+ + OH→ or more simply H 2 O → H+ + OHAt equilibrium → [H+] = [OH-] = 1. 00 x 10 -7 mol/L The water equilibrium constant, Kw, is called the ion product constant for water. Kw = [H+][OH-] = 1. 00 x 10 -14 T = 25 o. C Kw = (1 x 10 -7) = 1. 00 x 10 -14 50

What is the concentration of (a) H+ and (b) OH- in a 0. 001 M HCl solution? HCl is 100% ionized. HCl → H+ + Cl[H+] = 1 x 10 -3 M Solve the Kw expression for [OH-]. Kw= [H+][OH-] = 1. 00 x 10 -14 51

What is the p. H of a 0. 010 M Na. OH solution? Na. OH is 100% ionized. Na. OH → Na+ + OH[OH-] = 1 x 10 -2 M Solve the Kw expression for [H+]. Kw= [H+][OH-] = 1. 00 x 10 -14 p. H = - log[H+] = - log(1. 0 x 10 -12) = 12 52
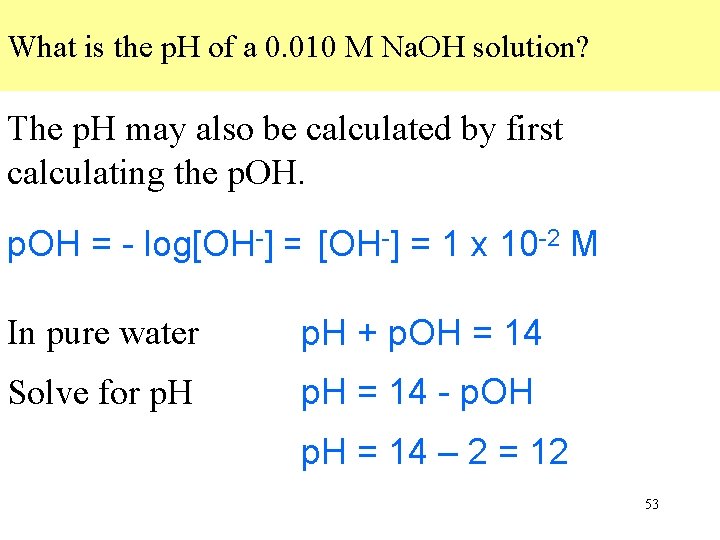
What is the p. H of a 0. 010 M Na. OH solution? The p. H may also be calculated by first calculating the p. OH. -] = 1 xx 10 p. OH = - log[OH-] = -[OH log(1. 0 10 -2 -2) = M 2 In pure water p. H + p. OH = 14 Solve for p. H = 14 - p. OH p. H = 14 – 2 = 12 53
![Relationship of H+ and OHConcentrations in Water Solutions [H+] 16. 1 [OH-] Kw p. Relationship of H+ and OHConcentrations in Water Solutions [H+] 16. 1 [OH-] Kw p.](http://slidetodoc.com/presentation_image_h2/4612942338364a08b2a9f9e3475a61f0/image-54.jpg)
Relationship of H+ and OHConcentrations in Water Solutions [H+] 16. 1 [OH-] Kw p. H p. OH 1. 00 x 10 -2 1. 00 x 10 -14 2. 00 1. 00 x 10 -4 1. 00 x 10 -10 1. 00 x 10 -14 4. 00 10. 00 2. 00 x 10 -6 5. 00 x 10 -9 1. 00 x 10 -14 5. 70 8. 30 1. 00 x 10 -7 1. 00 x 10 -14 7. 00 1. 00 x 10 -9 1. 00 x 10 -5 1. 00 x 10 -14 9. 00 54

16. 11 Ionization Constants 55

• Strong acids are essentially 100% ionized and do not have ionization constants. • Weak acids are slightly ionized and do have ionization constants. 56

Ka 57
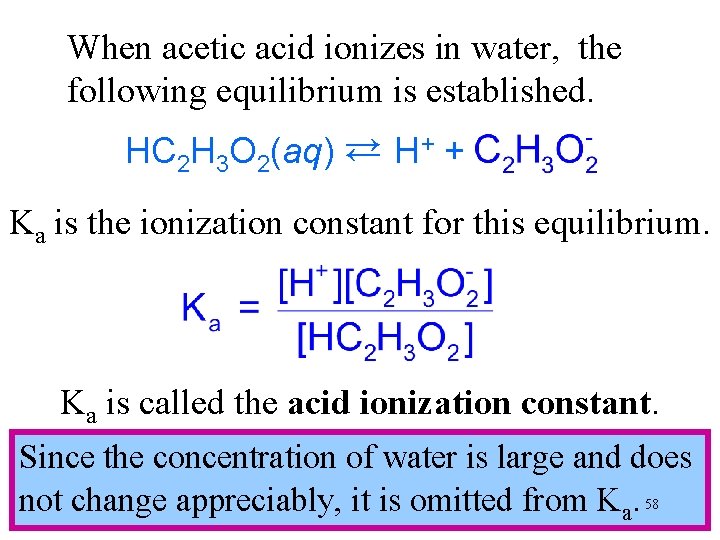
When acetic acid ionizes in water, the following equilibrium is established. HC H O (aq) → H+ + C H O 3 2 → 2 2 3 2 Ka is the ionization constant for this equilibrium. Ka is called the acid ionization constant. Since the concentration of water is large and does not change appreciably, it is omitted from Ka. 58
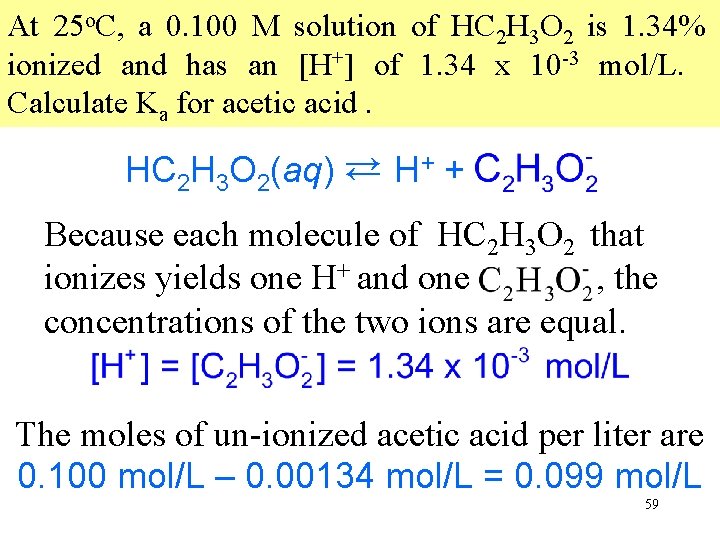
At 25 o. C, a 0. 100 M solution of HC 2 H 3 O 2 is 1. 34% ionized and has an [H+] of 1. 34 x 10 -3 mol/L. Calculate Ka for acetic acid. HC 2 H 3 O 2(aq) → H+ + C 2 H 3 O 2 → Because each molecule of HC 2 H 3 O 2 that ionizes yields one H+ and one C 2 H 3 O 2 , the concentrations of the two ions are equal. The moles of un-ionized acetic acid per liter are 0. 100 mol/L – 0. 00134 mol/L = 0. 099 mol/L 59
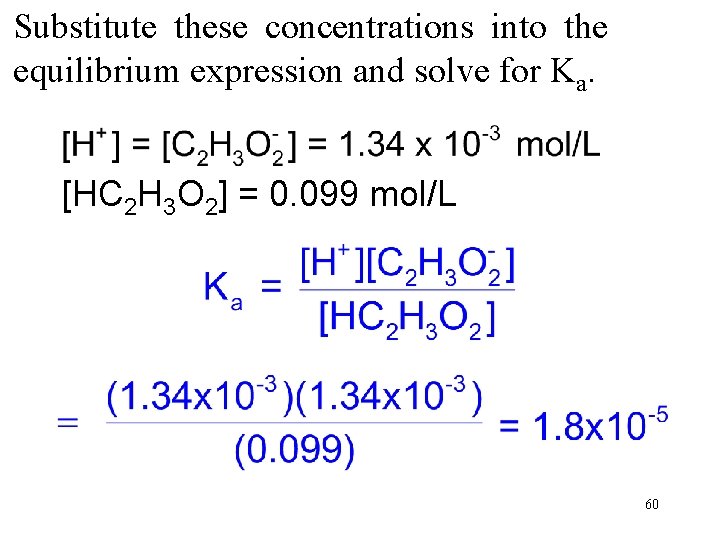
Substitute these concentrations into the equilibrium expression and solve for Ka. [HC 2 H 3 O 2] = 0. 099 mol/L 60
![What is the [H+] in a 0. 50 M HC 2 H 3 O What is the [H+] in a 0. 50 M HC 2 H 3 O](http://slidetodoc.com/presentation_image_h2/4612942338364a08b2a9f9e3475a61f0/image-61.jpg)
What is the [H+] in a 0. 50 M HC 2 H 3 O 2 solution? The ionization constant, Ka, for HC 2 H 3 O 2 is 1. 8 x 10 -5. The equilibrium expression and Ka for HC 2 H 3 O 2 are HC 2 H 3 O 2(aq) → H+ + C 2 H 3 O 2 → +]O Because each molecule H Letof. YHC = [H 2 3 =2 that + and one C H O , the ionizes yields one H 2 – Y. [HC 2 H 3 O 2] at equilibrium 2 is 30. 50 concentrations of the two ions are equal. 61
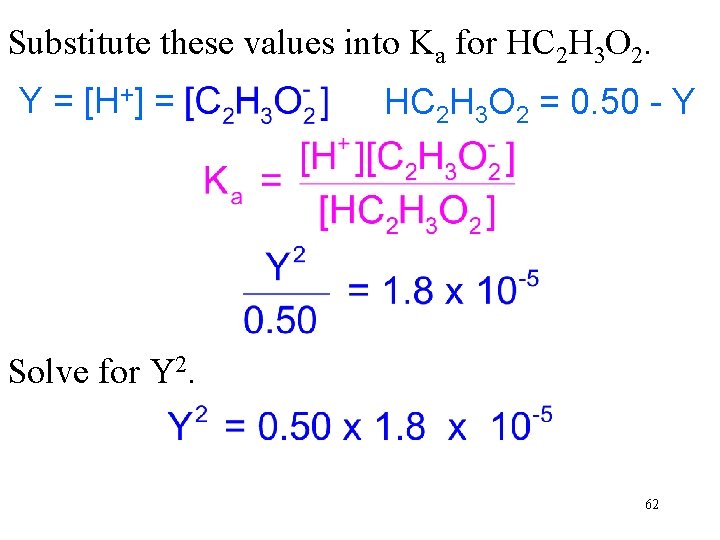
Substitute these values into Ka for HC 2 H 3 O 2. Y = [H+] = HC 2 H 3 O 2 = 0. 50 - Y Solve for Y 2. Assume Y is small compared to Then 0. 50 – Y 0. 50 -Y. 62
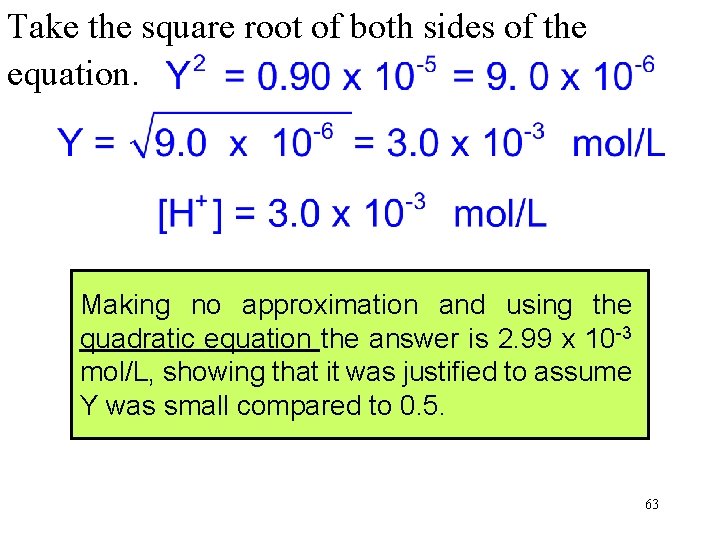
Take the square root of both sides of the equation. Making no approximation and using the quadratic equation the answer is 2. 99 x 10 -3 mol/L, showing that it was justified to assume Y was small compared to 0. 5. 63

Calculate the percent ionization in a 0. 50 M HC 2 H 3 O 2 solution. The ionization of a weak acid is given by HA → H+ + A→ The percent ionization is given by 65
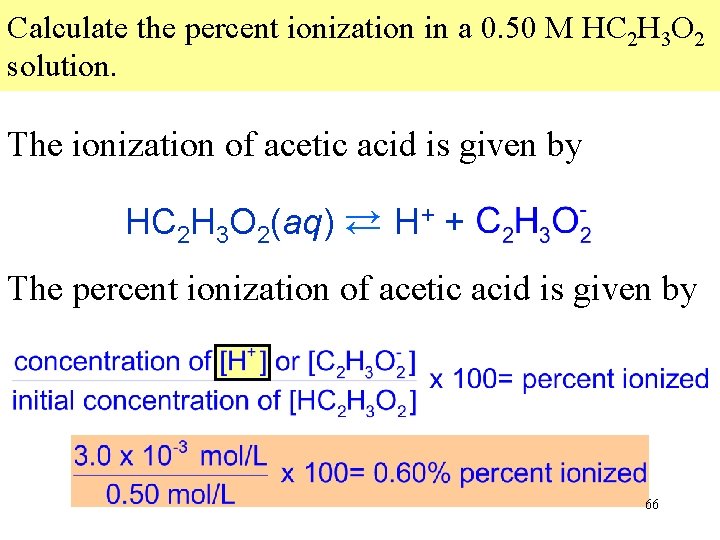
Calculate the percent ionization in a 0. 50 M HC 2 H 3 O 2 solution. The ionization of acetic acid is given by HC 2 H 3 O 2(aq) → H+ + C 2 H 3 O 2 → The percent ionization of acetic acid is given by [H+] was previously calculated as 3. 0 x 10 -3 mol/L 66
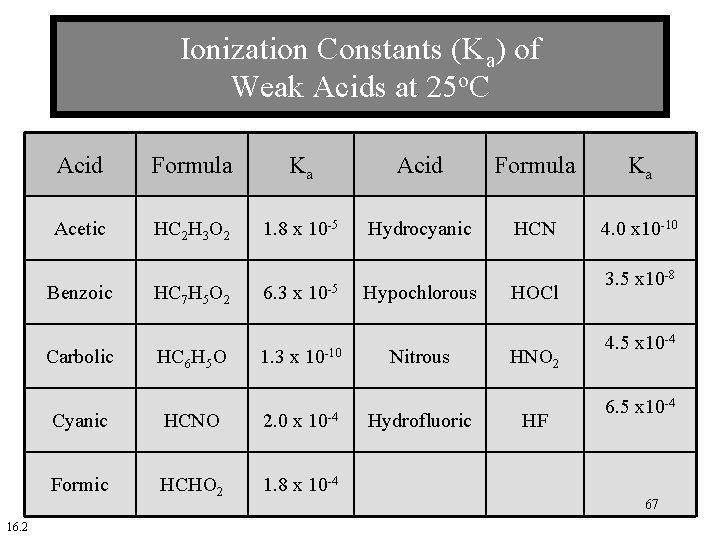
Ionization Constants (Ka) of Weak Acids at 25 o. C Acid Formula Ka Acetic HC 2 H 3 O 2 1. 8 x 10 -5 Hydrocyanic HCN 4. 0 x 10 -10 Benzoic Carbolic 16. 2 HC 7 H 5 O 2 HC 6 H 5 O 6. 3 x 10 -5 1. 3 x 10 -10 Cyanic HCNO 2. 0 x 10 -4 Formic HCHO 2 1. 8 x 10 -4 Hypochlorous Nitrous Hydrofluoric HOCl HNO 2 HF 3. 5 x 10 -8 4. 5 x 10 -4 67

16. 12 Solubility Product Constant 68

chemical equilibrium A dynamic state The in state which in The solubility product constant, Ksp, is two orthemore which rate opposing of the forward processes reaction are the equilibrium constant of a slightly taking the equals place rateatofthe thesame reverse time reaction and at in thea soluble salt. same rate. change. chemical 69
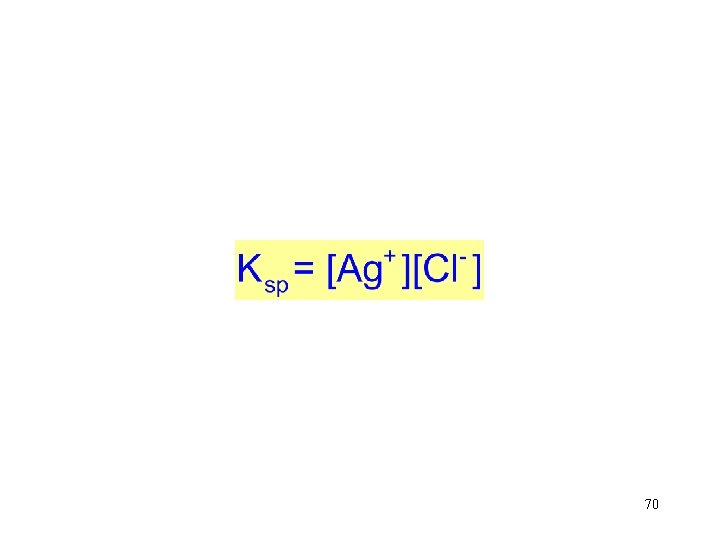
Silver chloride is in equilibrium with its ions in aqueous solution. Ag. Cl(s) → Ag+(aq) + Cl-(aq) → The equilibrium constant is The product of Keq and [Ag. Cl(s) is a constant. Rearrange The amount concentration of solid Ag. Cl of does solidnot Ag. Cl affect is a the constant. equilibrium. 70
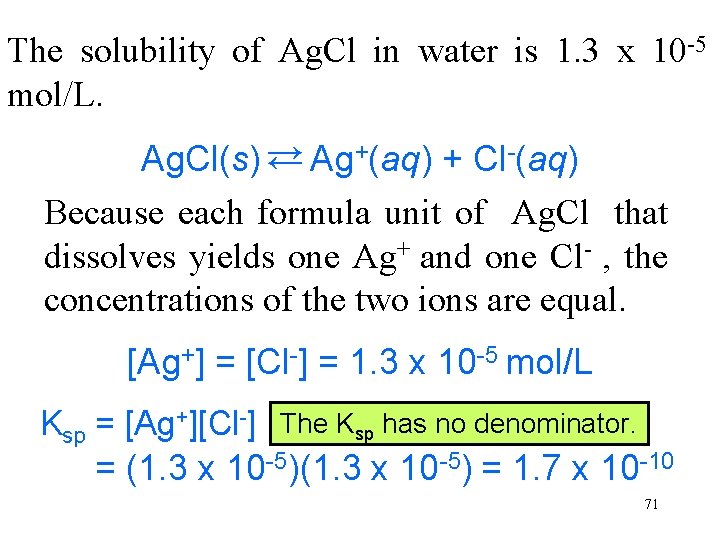
The solubility of Ag. Cl in water is 1. 3 x 10 -5 mol/L. Ag. Cl(s) → Ag+(aq) + Cl-(aq) Because each formula unit of Ag. Cl that dissolves yields one Ag+ and one Cl- , the concentrations of the two ions are equal. → [Ag+] = [Cl-] = 1. 3 x 10 -5 mol/L Ksp = [Ag+][Cl-] The Ksp has no denominator. = (1. 3 x 10 -5) = 1. 7 x 10 -10 71

When the product of the molar concentration of the ions in solution (each raised to its proper power) is greater than the Ksp for that substance, precipitation will occur. If the ion product is less than the Ksp value no precipitation will occur. 72
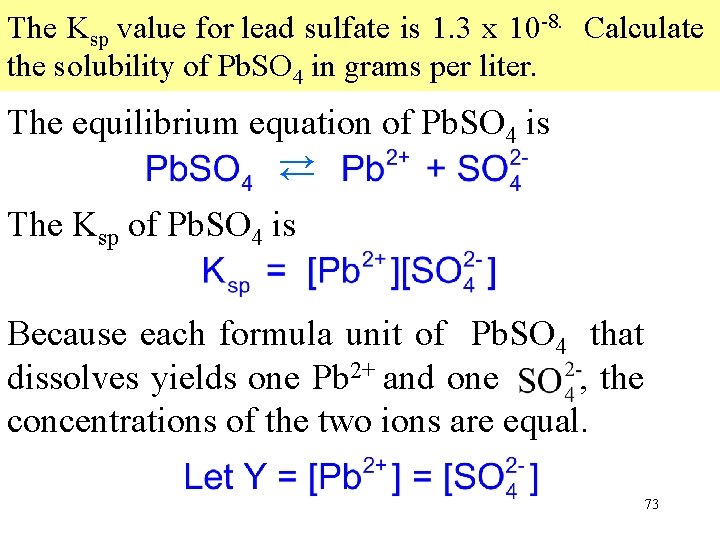
The Ksp value for lead sulfate is 1. 3 x 10 -8. Calculate the solubility of Pb. SO 4 in grams per liter. The equilibrium equation of Pb. SO 4 is → → The Ksp of Pb. SO 4 is Because each formula unit of Pb. SO 4 that dissolves yields one Pb 2+ and one , the concentrations of the two ions are equal. 73
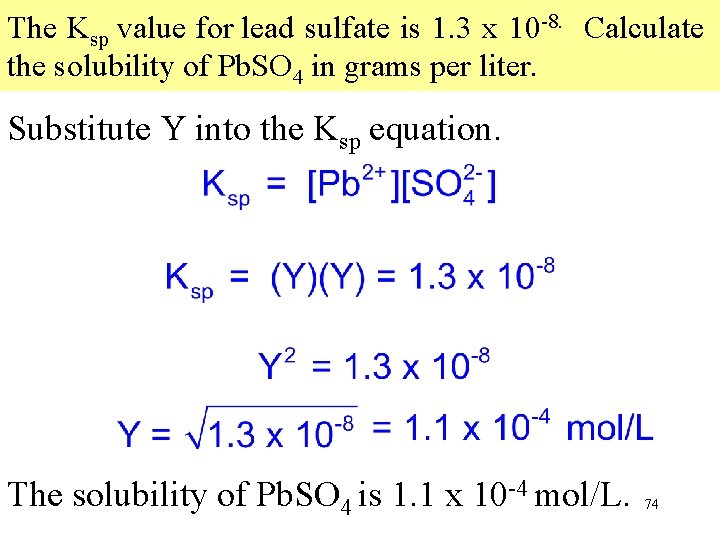
The Ksp value for lead sulfate is 1. 3 x 10 -8. Calculate the solubility of Pb. SO 4 in grams per liter. Substitute Y into the Ksp equation. The solubility of Pb. SO 4 is 1. 1 x 10 -4 mol/L. 74

The Ksp value for lead sulfate is 1. 3 x 10 -8. Calculate the solubility of Pb. SO 4 in grams per liter. Convert mol/L to grams/L. The molar mass of Pb. SO 4 is 303. 3 g/mol. The solubility of Pb. SO 4 is 3. 3 x 10 -2 g/L. 75

The Common Ion Effect 76

A shift in the equilibrium position upon An ion added to a solution already addition of an ion already contained in containing that ion is called a common the solution is known as the common ion. effect. 77
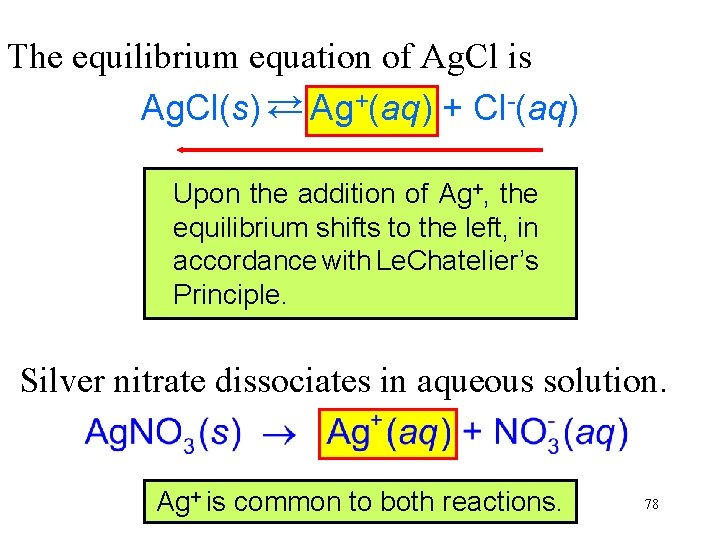
The equilibrium equation of Ag. Cl is Ag. Cl(s) → Ag+(aq) + Cl-(aq) → Upon the addition of Ag+, the equilibrium shifts to the left, in accordance with Le. Chatelier’s Principle. Silver nitrate dissociates in aqueous solution. Ag+ is common to both reactions. 78
![Silver nitrate is added to a saturated Ag. Cl solution until the [Ag+]=0. 10 Silver nitrate is added to a saturated Ag. Cl solution until the [Ag+]=0. 10](http://slidetodoc.com/presentation_image_h2/4612942338364a08b2a9f9e3475a61f0/image-78.jpg)
Silver nitrate is added to a saturated Ag. Cl solution until the [Ag+]=0. 10 M. What will be the [Cl-] remaining in solution? Use Ksp of Ag. Cl to determine [Cl-]. Substitute [Ag+] into the Ksp. Solve for [Cl-]. This In the is an absence example of of Ag. NO the 3 common , [Cl-] =1. 3 ionx effect. 10 -5. 79
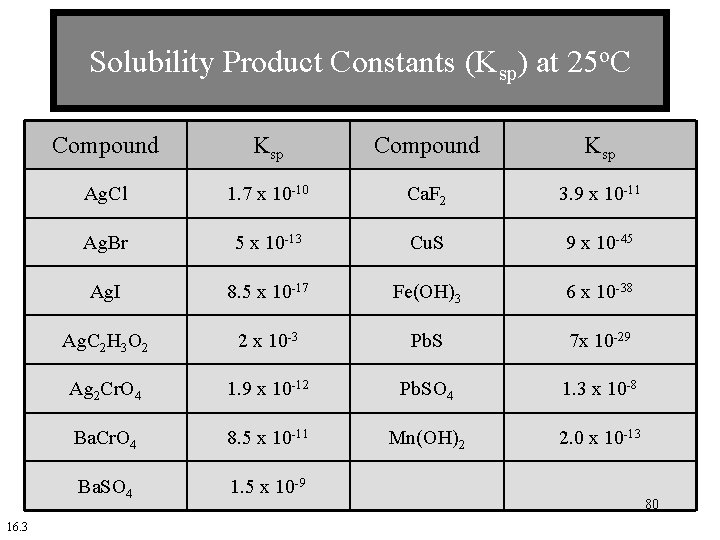
Solubility Product Constants (Ksp) at 25 o. C 16. 3 Compound Ksp Ag. Cl 1. 7 x 10 -10 Ca. F 2 3. 9 x 10 -11 Ag. Br 5 x 10 -13 Cu. S 9 x 10 -45 Ag. I 8. 5 x 10 -17 Fe(OH)3 6 x 10 -38 Ag. C 2 H 3 O 2 2 x 10 -3 Pb. S 7 x 10 -29 Ag 2 Cr. O 4 1. 9 x 10 -12 Pb. SO 4 1. 3 x 10 -8 Ba. Cr. O 4 8. 5 x 10 -11 Mn(OH)2 2. 0 x 10 -13 Ba. SO 4 1. 5 x 10 -9 80

16. 13 Acid-Base Properties of Salts 81

hydrolysis is the term used for the general reaction in which a water molecule is split. 82
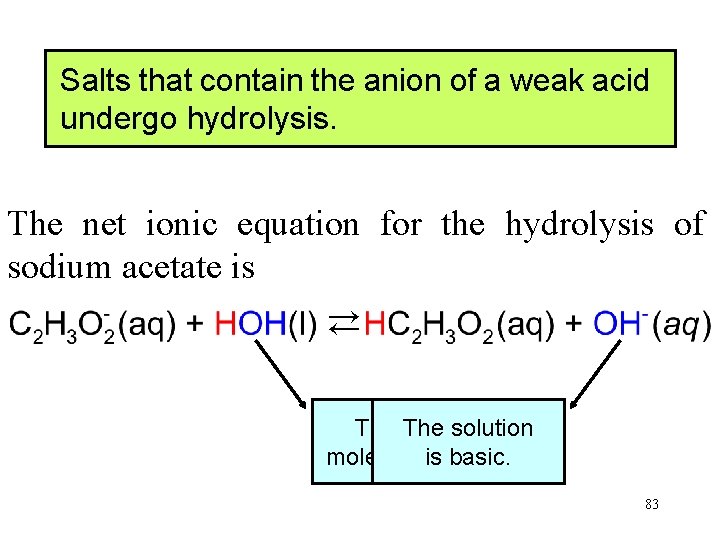
Salts that contain the anion of a weak acid undergo hydrolysis. The net ionic equation for the hydrolysis of sodium acetate is → → The water The solution molecule is splits. basic. 83

Salts that contain the cation of a weak base undergo hydrolysis. The net ionic equation for the hydrolysis of ammonium chloride is → → Thesolution water molecule is acidic. splits. 84
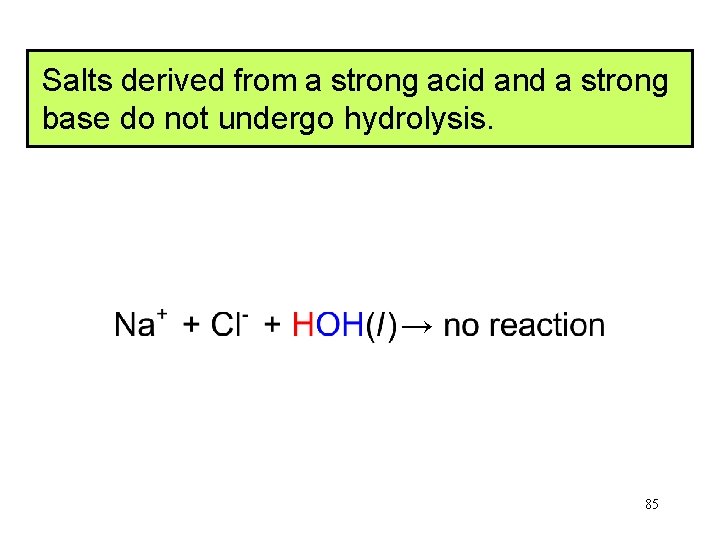
Salts derived from a strong acid and a strong base do not undergo hydrolysis. → 85

Ionic Composition of Salts and the Nature of the Aqueous Solutions They Form Type of salt Nature of Aqueous Solution Examples Weak base-strong acid Acid NH 4 Cl, NH 4 NO 3 Strong base-weak acid Basic Na. C 2 H 3 O 2 Weak base-weak acid Depends on the salt NH 4 C 2 H 3 O 2, NH 4 NO 2 Strong base-strong acid Neutral Na. Cl, KBr 86 16. 4

16. 14 Buffer Solutions: The Control of p. H 87

A buffer solution resists changes in p. H when diluted or when small amounts of acid or base are added. 88

A weak acid mixed with a salt of its conjugate base forms a buffer solution. Sodium acetate, when mixed with acetic acid, forms a buffer solution. 89
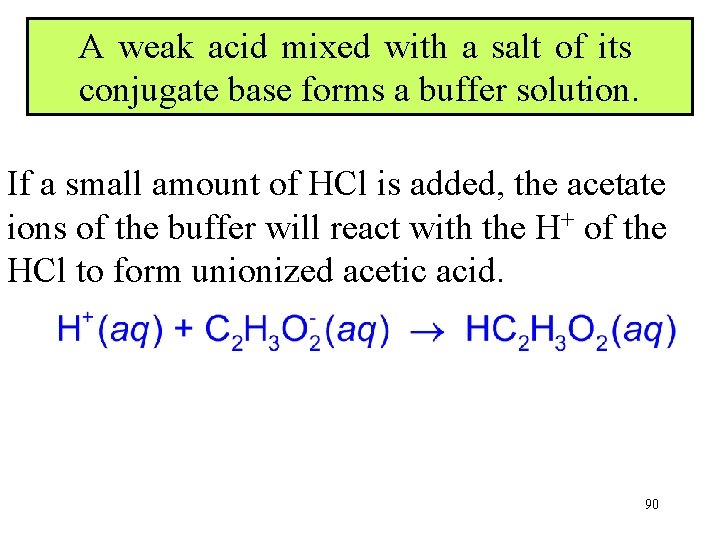
A weak acid mixed with a salt of its conjugate base forms a buffer solution. If a small amount of HCl is added, the acetate ions of the buffer will react with the H+ of the HCl to form unionized acetic acid. 90

A weak acid mixed with a salt of its conjugate base forms a buffer solution. If a small amount of Na. OH is added, the acetic acid molecules of the buffer will react with the OH- of the Na. OH to form water. 91

Changes in p. H Caused by the Addition of HCl and Na. OH Solution H 2 O (1000 m. L) p. H Change in p. H 7 H 2 O + 0. 010 mol HCl 2 5 H 2 O + 0. 010 mol Na. OH 12 5 0. 10 M HC 2 H 3 O 2 + 0. 10 M Na. C 2 H 3 O 2 4. 74 – Buffer + 0. 010 mol HCl 4. 66 0. 08 Buffer + 0. 010 mol Na. OH 4. 83 0. 09 Buffer solution (1000 m. L) 92 16. 5

93
- Slides: 92