Chemical Equilibrium Chapter 14 Chemical Equilibrium 14 1
Chemical Equilibrium

Chapter 14 Chemical Equilibrium • 14. 1 the concept of equilibrium and the equilibrium constant • 14. 4 writing equilibrium constant expression • 14. 4 what does the equilibrium constant tell us • 14. 5 factors that effect chemical equilibrium
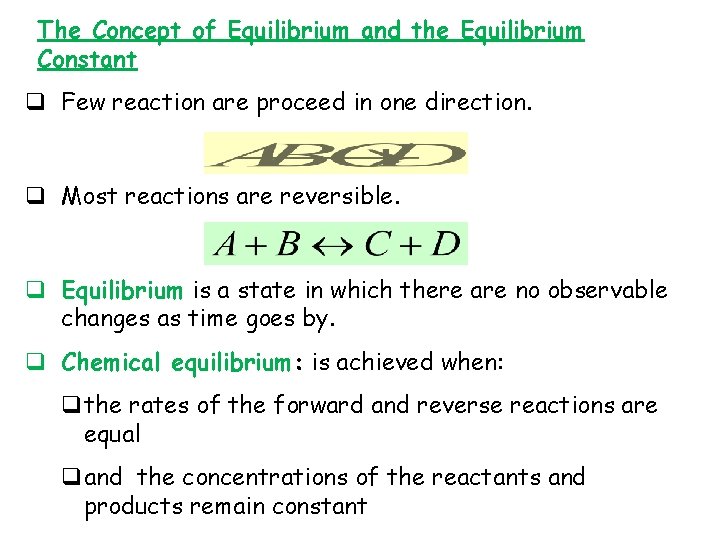
The Concept of Equilibrium and the Equilibrium Constant q Few reaction are proceed in one direction. q Most reactions are reversible. q Equilibrium is a state in which there are no observable changes as time goes by. q Chemical equilibrium: is achieved when: qthe rates of the forward and reverse reactions are equal qand the concentrations of the reactants and products remain constant
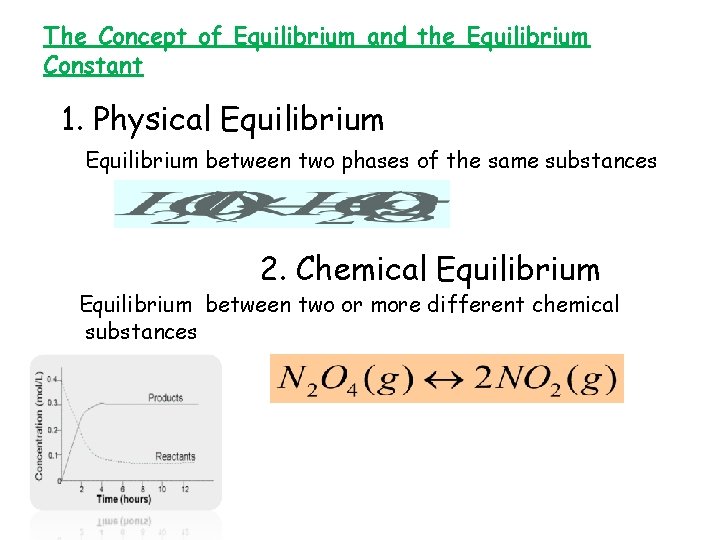
The Concept of Equilibrium and the Equilibrium Constant 1. Physical Equilibrium between two phases of the same substances 2. Chemical Equilibrium between two or more different chemical substances
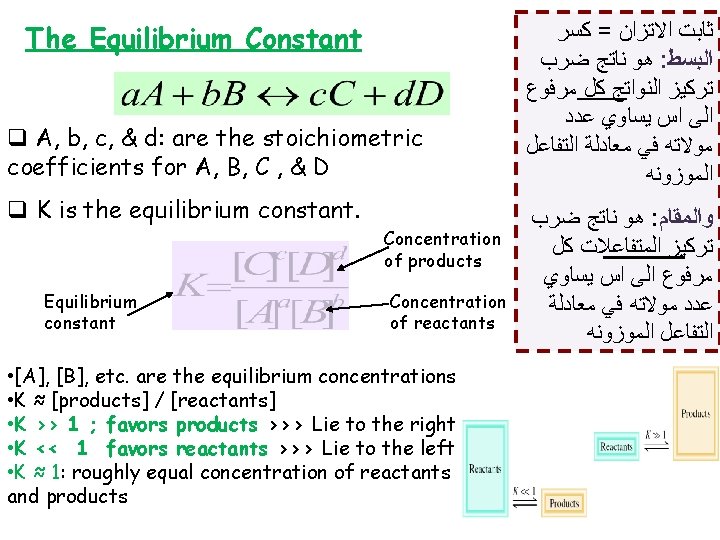
The Equilibrium Constant q A, b, c, & d: are the stoichiometric coefficients for A, B, C , & D q K is the equilibrium constant. Concentration of products Equilibrium constant Concentration of reactants • [A], [B], etc. are the equilibrium concentrations • K ≈ [products] / [reactants] • K >> 1 ; favors products >>> Lie to the right • K << 1 favors reactants >>> Lie to the left • K ≈ 1: roughly equal concentration of reactants and products ﺛﺎﺑﺖ ﺍﻻﺗﺰﺍﻥ = ﻛﺴﺮ ﻫﻮ ﻧﺎﺗﺞ ﺿﺮﺏ : ﺍﻟﺒﺴﻂ ﺗﺮﻛﻴﺰ ﺍﻟﻨﻮﺍﺗﺞ ﻛﻞ ﻣﺮﻓﻮﻉ ﺍﻟﻰ ﺍﺱ ﻳﺴﺎﻭﻱ ﻋﺪﺩ ﻣﻮﻻﺗﻪ ﻓﻲ ﻣﻌﺎﺩﻟﺔ ﺍﻟﺘﻔﺎﻋﻞ ﺍﻟﻤﻮﺯﻭﻧﻪ ﻫﻮ ﻧﺎﺗﺞ ﺿﺮﺏ : ﻭﺍﻟﻤﻘﺎﻡ ﺗﺮﻛﻴﺰ ﺍﻟﻤﺘﻔﺎﻋﻼﺕ ﻛﻞ ﻣﺮﻓﻮﻉ ﺍﻟﻰ ﺍﺱ ﻳﺴﺎﻭﻱ ﻋﺪﺩ ﻣﻮﻻﺗﻪ ﻓﻲ ﻣﻌﺎﺩﻟﺔ ﺍﻟﺘﻔﺎﻋﻞ ﺍﻟﻤﻮﺯﻭﻧﻪ

The Equilibrium Constant • K is a constant at a given temperature • Solids and pure liquid drop out of the expression & water drops out when the solvent is water • K has no unit
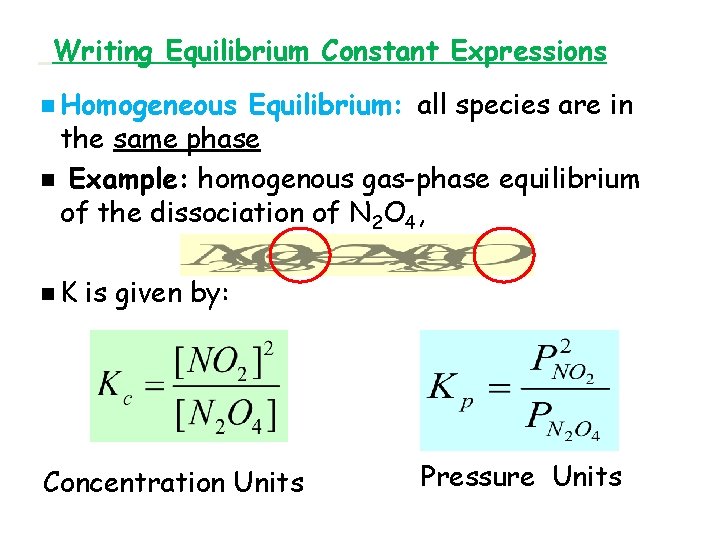
Writing Equilibrium Constant Expressions n Homogeneous Equilibrium: all species are in the same phase n Example: homogenous gas-phase equilibrium of the dissociation of N 2 O 4, n. K is given by: Concentration Units Pressure Units
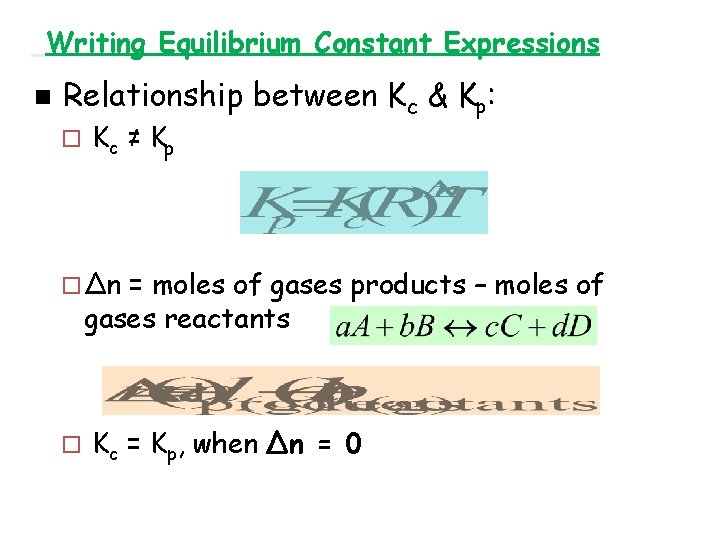
Writing Equilibrium Constant Expressions n Relationship between Kc & Kp : ¨ Kc ≠ Kp ¨ ∆n = moles of gases products – moles of gases reactants ¨ Kc = Kp, when ∆n = 0
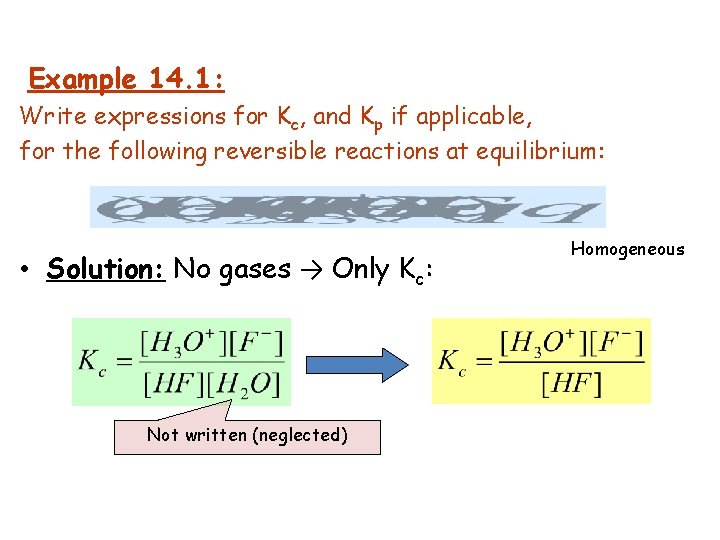
Example 14. 1: Write expressions for Kc, and Kp if applicable, for the following reversible reactions at equilibrium: • Solution: No gases → Only Kc: Not written (neglected) Homogeneous
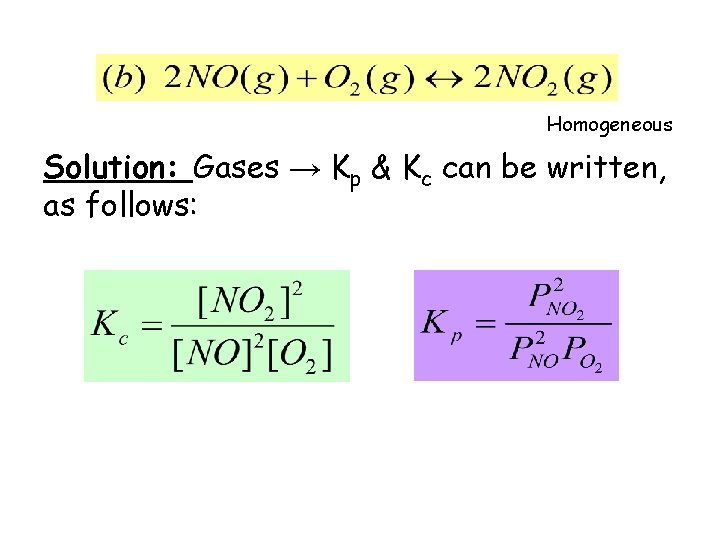
Homogeneous Solution: Gases → Kp & Kc can be written, as follows:
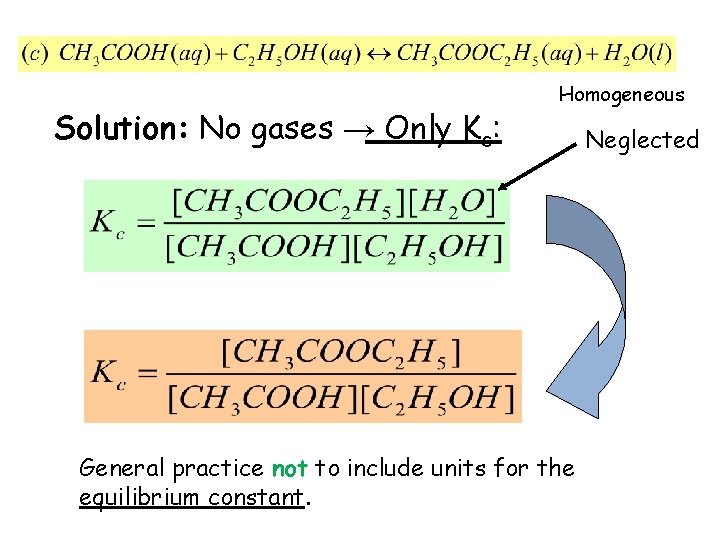
Solution: No gases → Only Kc: Homogeneous General practice not to include units for the equilibrium constant. Neglected

Example 14. 2: The following equilibrium process has been studied at 230 o. C: Homogeneous In one experiment, the concentrations of the reacting species at equilibrium are found to be [NO] = 0. 0542 M, [O 2] = 0. 127 M, and [NO 2] = 15. 5 M. Calculate the equilibrium constant (Kc) of this reaction at this temperature. Solution: Large magnitude favors products >>> Lie to the right
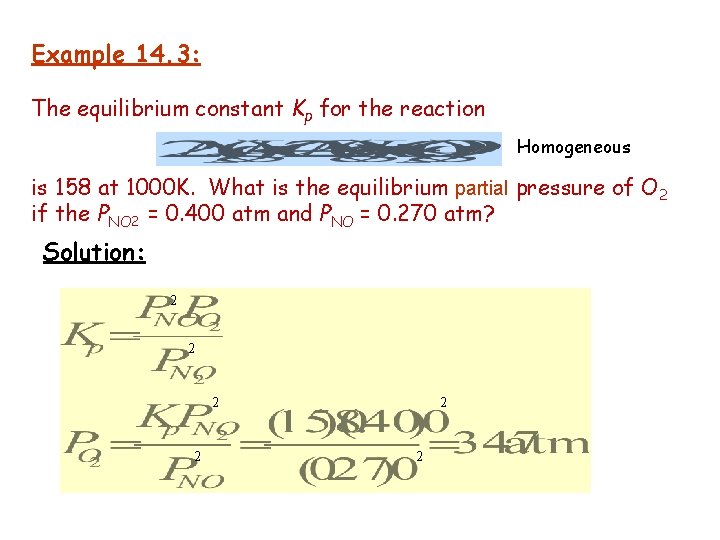
Example 14. 3: The equilibrium constant Kp for the reaction Homogeneous is 158 at 1000 K. What is the equilibrium partial pressure of O 2 if the PNO 2 = 0. 400 atm and PNO = 0. 270 atm? Solution: 2 2 2
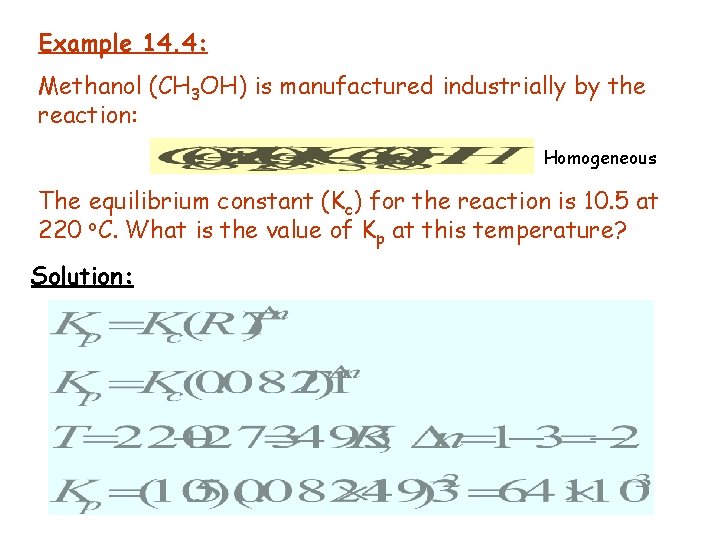
Example 14. 4: Methanol (CH 3 OH) is manufactured industrially by the reaction: Homogeneous The equilibrium constant (Kc) for the reaction is 10. 5 at 220 o. C. What is the value of Kp at this temperature? Solution:
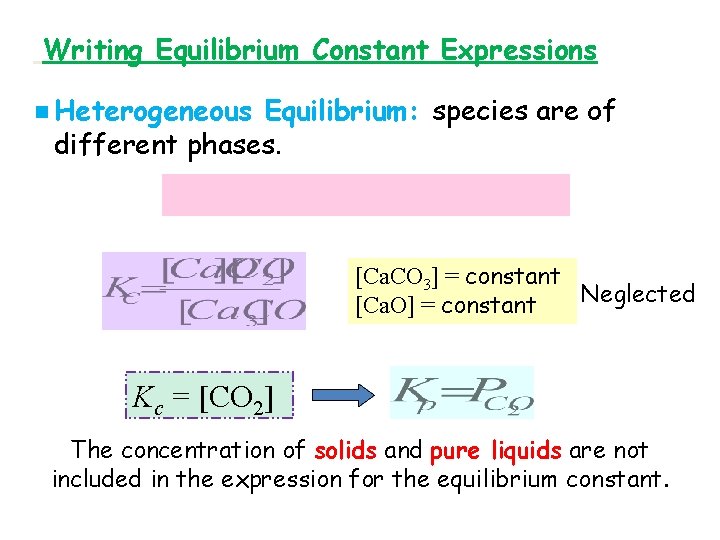
Writing Equilibrium Constant Expressions n Heterogeneous Equilibrium: species are of different phases. [Ca. CO 3] = constant Neglected [Ca. O] = constant Kc = [CO 2] The concentration of solids and pure liquids are not included in the expression for the equilibrium constant.
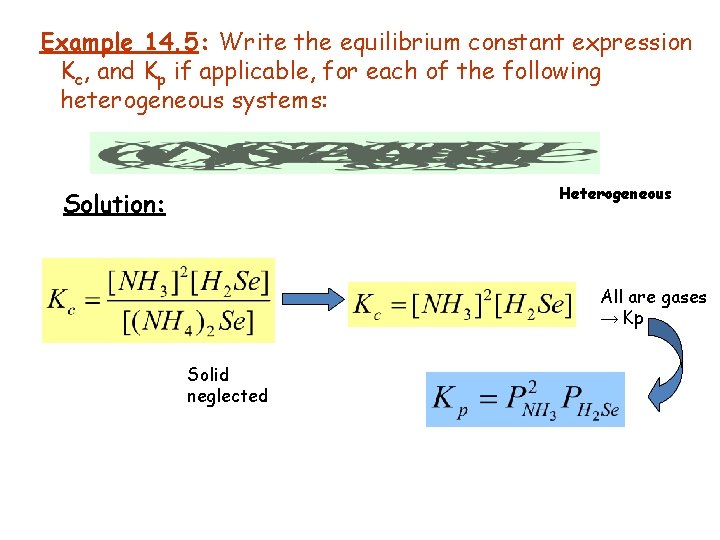
Example 14. 5: Write the equilibrium constant expression Kc, and Kp if applicable, for each of the following heterogeneous systems: Heterogeneous Solution: All are gases → Kp Solid neglected
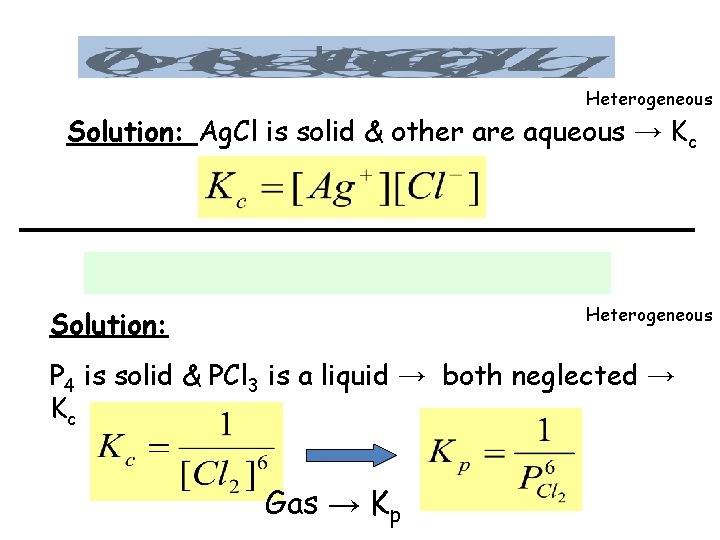
Heterogeneous Solution: Ag. Cl is solid & other are aqueous → Kc Heterogeneous Solution: P 4 is solid & PCl 3 is a liquid → both neglected → Kc Gas → Kp
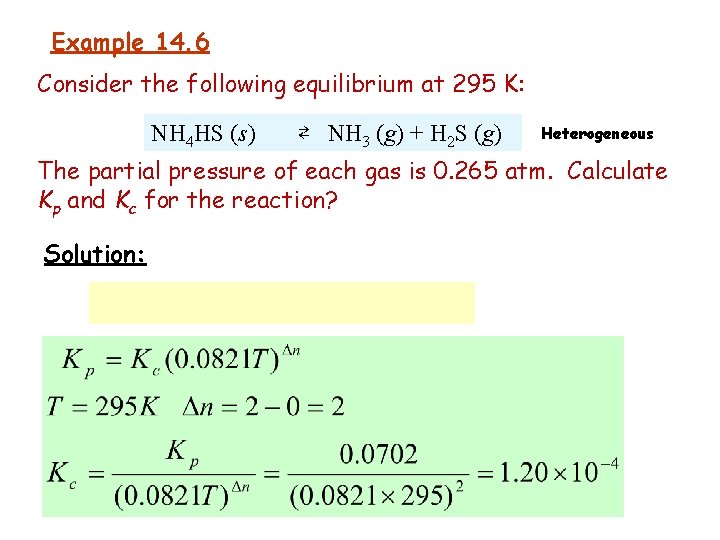
Example 14. 6 Consider the following equilibrium at 295 K: NH 4 HS (s) ⇄ NH 3 (g) + H 2 S (g) Heterogeneous The partial pressure of each gas is 0. 265 atm. Calculate Kp and Kc for the reaction? Solution:
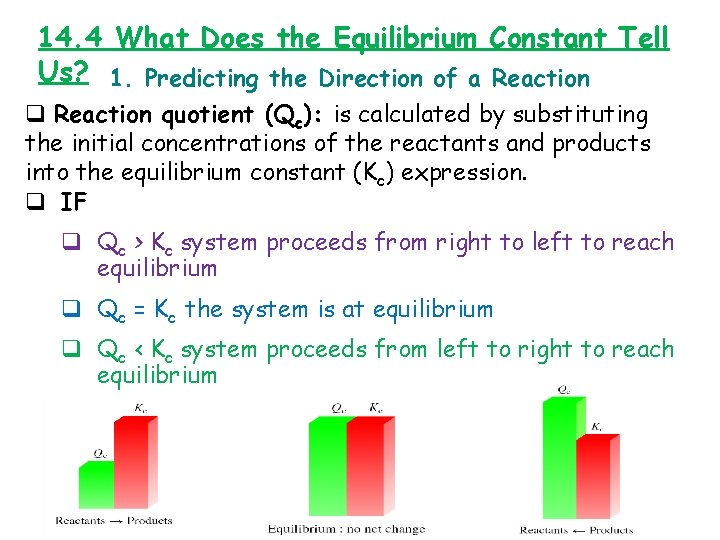
14. 4 What Does the Equilibrium Constant Tell Us? 1. Predicting the Direction of a Reaction quotient (Qc): is calculated by substituting the initial concentrations of the reactants and products into the equilibrium constant (Kc) expression. q IF q Qc > Kc system proceeds from right to left to reach equilibrium q Qc = Kc the system is at equilibrium q Qc < Kc system proceeds from left to right to reach equilibrium
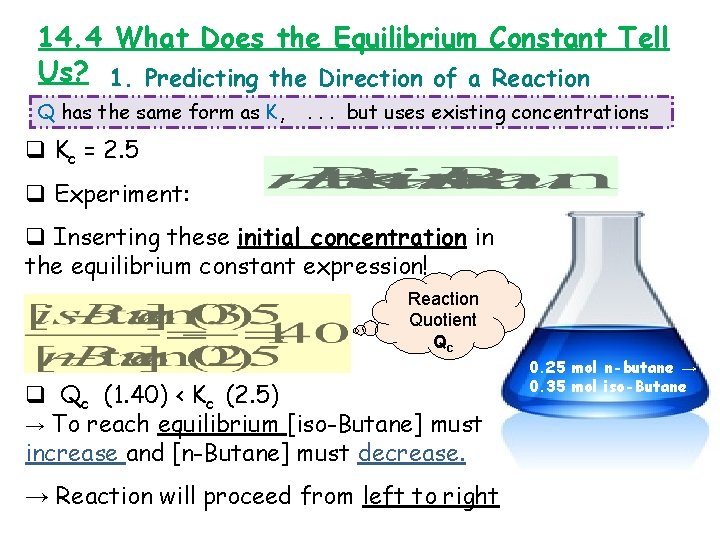
14. 4 What Does the Equilibrium Constant Tell Us? 1. Predicting the Direction of a Reaction Q has the same form as K, . . . but uses existing concentrations q Kc = 2. 5 q Experiment: q Inserting these initial concentration in the equilibrium constant expression! Reaction Quotient Qc q Qc (1. 40) < Kc (2. 5) → To reach equilibrium [iso-Butane] must increase and [n-Butane] must decrease. → Reaction will proceed from left to right 0. 25 mol n-butane → 0. 35 mol iso-Butane
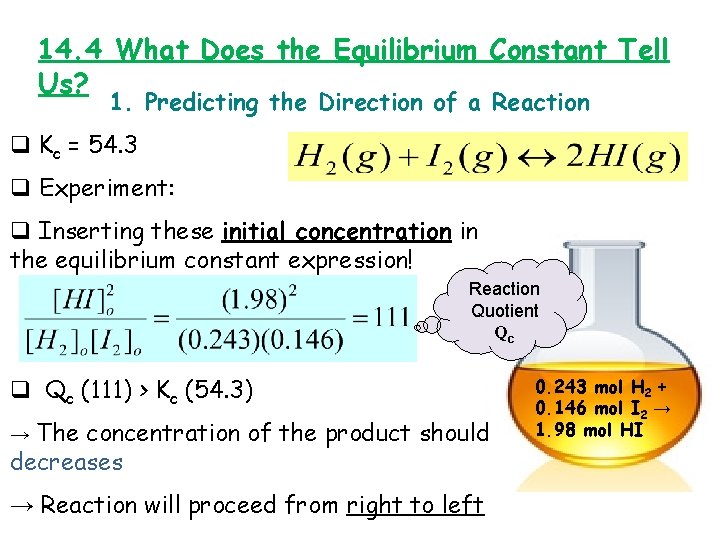
14. 4 What Does the Equilibrium Constant Tell Us? 1. Predicting the Direction of a Reaction q Kc = 54. 3 q Experiment: q Inserting these initial concentration in the equilibrium constant expression! Reaction Quotient Qc q Qc (111) > Kc (54. 3) → The concentration of the product should decreases → Reaction will proceed from right to left 0. 243 mol H 2 + 0. 146 mol I 2 → 1. 98 mol HI
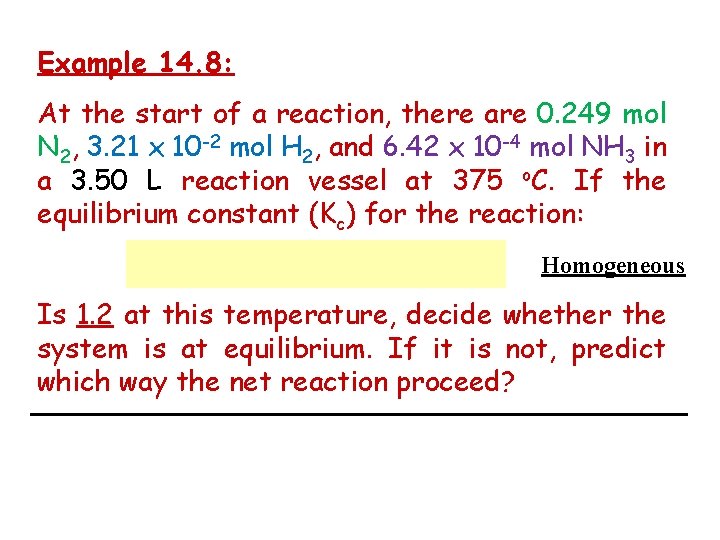
Example 14. 8: At the start of a reaction, there are 0. 249 mol N 2, 3. 21 x 10 -2 mol H 2, and 6. 42 x 10 -4 mol NH 3 in a 3. 50 L reaction vessel at 375 o. C. If the equilibrium constant (Kc) for the reaction: Homogeneous Is 1. 2 at this temperature, decide whether the system is at equilibrium. If it is not, predict which way the net reaction proceed?
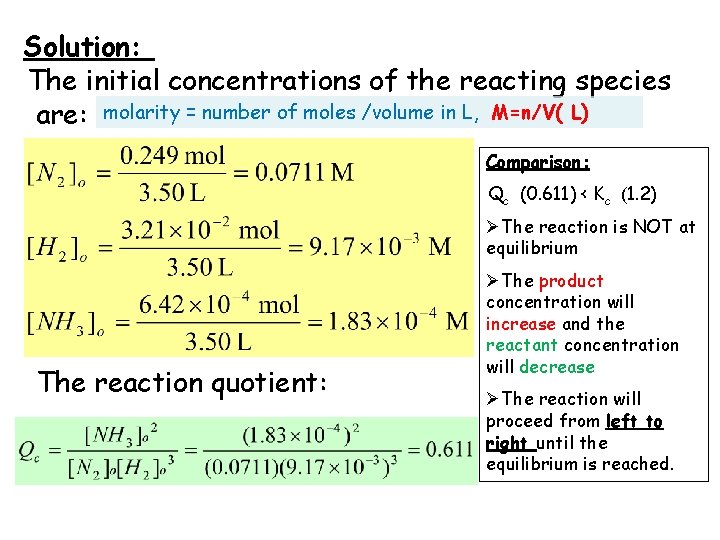
Solution: The initial concentrations of the reacting species are: molarity = number of moles /volume in L, M=n/V( L) Comparison: Qc (0. 611) < Kc (1. 2) ØThe reaction is NOT at equilibrium The reaction quotient: ØThe product concentration will increase and the reactant concentration will decrease ØThe reaction will proceed from left to right until the equilibrium is reached.
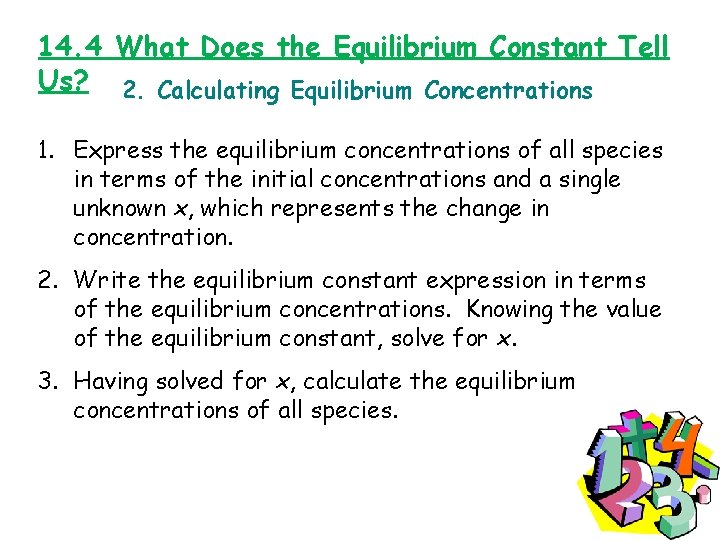
14. 4 What Does the Equilibrium Constant Tell Us? 2. Calculating Equilibrium Concentrations 1. Express the equilibrium concentrations of all species in terms of the initial concentrations and a single unknown x, which represents the change in concentration. 2. Write the equilibrium constant expression in terms of the equilibrium concentrations. Knowing the value of the equilibrium constant, solve for x. 3. Having solved for x, calculate the equilibrium concentrations of all species.
![example Kc = 24 Step 1 Initial Change At equilibrium Step 2 [cis-stilbene] 0. example Kc = 24 Step 1 Initial Change At equilibrium Step 2 [cis-stilbene] 0.](http://slidetodoc.com/presentation_image_h/dd716009c85a4b57e053f1ea96fd03e5/image-25.jpg)
example Kc = 24 Step 1 Initial Change At equilibrium Step 2 [cis-stilbene] 0. 85 -x 0. 85 -x [trans-stilbene] 0 +x x
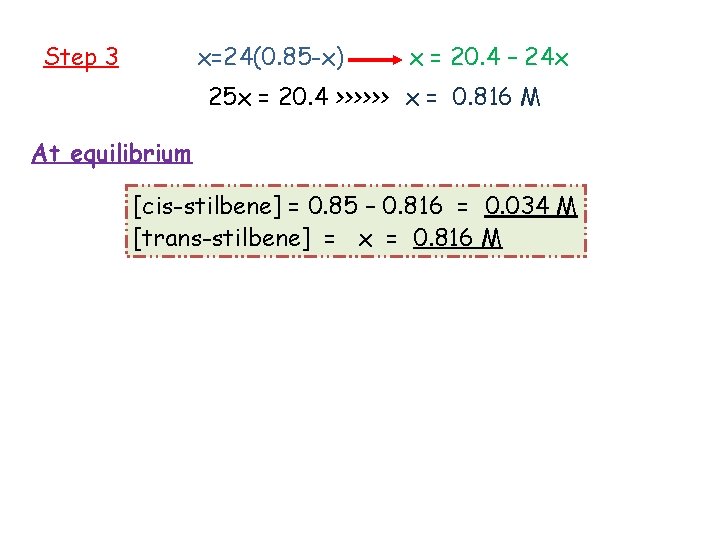
Step 3 x=24(0. 85 -x) x = 20. 4 – 24 x 25 x = 20. 4 >>>>>> x = 0. 816 M At equilibrium [cis-stilbene] = 0. 85 – 0. 816 = 0. 034 M [trans-stilbene] = x = 0. 816 M
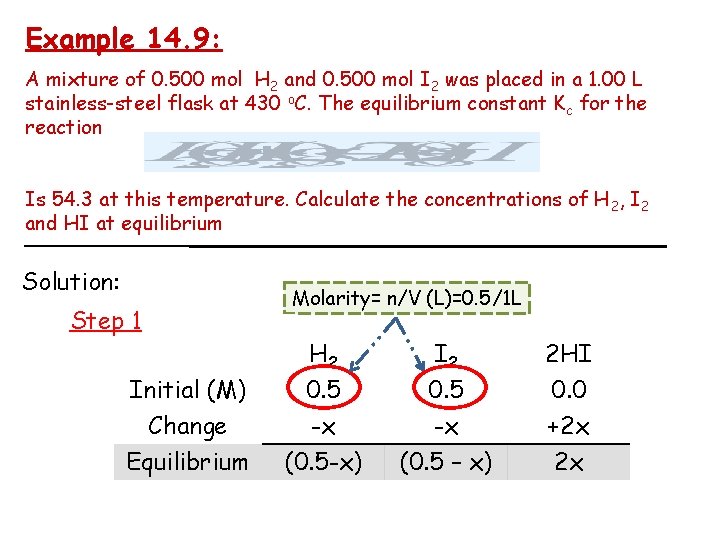
Example 14. 9: A mixture of 0. 500 mol H 2 and 0. 500 mol I 2 was placed in a 1. 00 L stainless-steel flask at 430 o. C. The equilibrium constant Kc for the reaction Is 54. 3 at this temperature. Calculate the concentrations of H 2, I 2 and HI at equilibrium Solution: Step 1 Initial (M) Change Equilibrium Molarity= n/V (L)=0. 5/1 L H 2 0. 5 -x (0. 5 -x) I 2 0. 5 -x (0. 5 – x) 2 HI 0. 0 +2 x 2 x
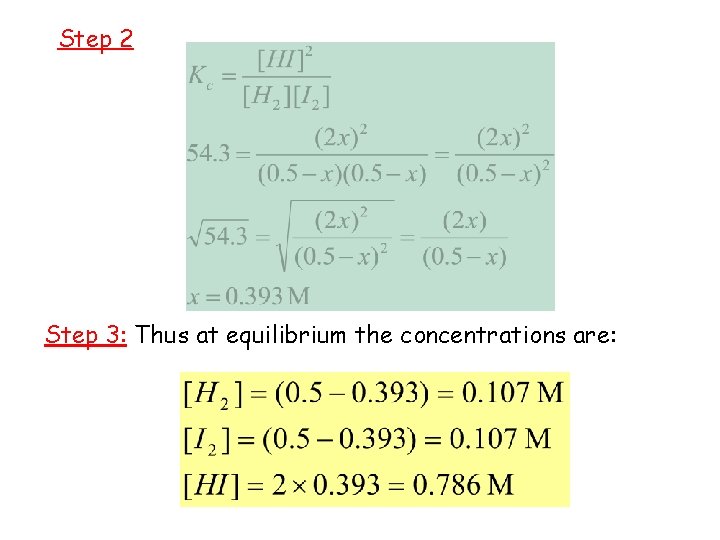
Step 2 Step 3: Thus at equilibrium the concentrations are:

Chemical Equilibrium
14. 5 Factors That Affect Chemical Equilibrium q Le Chatelier’s principle , If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position. ﻣﺒﺪﺃ ﻟﻮﺷﺎﺗﻠﻴﻴﻪ q factors that effect chemical equilibrium ﻋﻨﺪﻣﺎ ﻧﺆﺜﺮ ﺑﻤﺆﺜﺮ ﺧﺎﺭﺟﻲ 1. Changes in Concentration , ﻋﻠﻰ ﻧﻈﺎﻡ ﻓﻲ ﺣﺎﻟﺔ ﺍﺗﺰﺍﻥ ﻓﺎﻥ ﺍﻟﻨﻈﺎﻡ ﺳﻮﻑ ﻳﻌﺪﻝ ﻣﻦ 2. Changes in Volume and Pressure ﻧﻔﺴﻪ ﻟﻜﻲ ﻳﻌﻮﺽ ﺍﻟﺘﺎﺛﻴﺮ ﺍﻟﺤﺎﺻﻞ )ﻳﺰﺍﺡ ﻓﻲ ﺍﺗﺠﺎﻩ ﻳﻘﻠﻞ 3. Changes in Temperature ﻣﻦ ﺗﺄﺜﻴﺮ ﻫﺬﺍ ﺍﻟﻤﺆﺜﺮ( ﻭﺑﺎﻟﺘﺎﻟﻲ 4. Adding a Catalyst ﻳﺼﻞ ﺍﻟﻰ ﺣﺎﻟﺔ ﺍﻻﺗﺰﺍﻥ ﻣﺮﻩ Change any factor may change the position & value of K, or it may has no effect. . ﺍﺧﺮﻯ
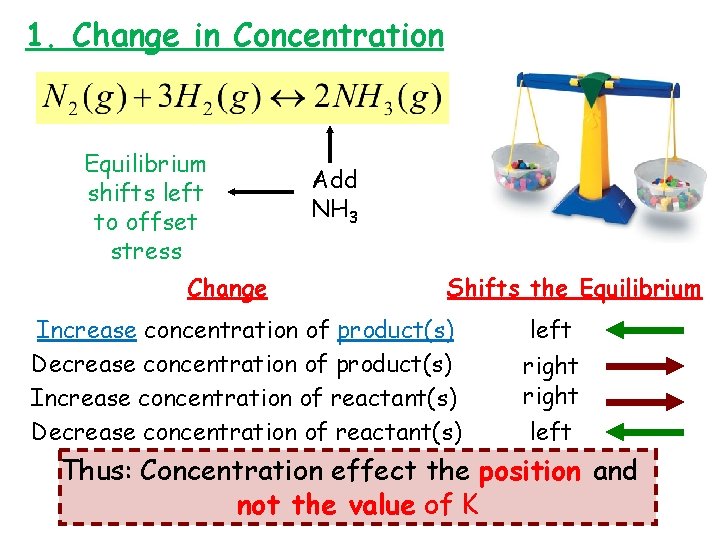
1. Change in Concentration Equilibrium shifts left to offset stress Change Add NH 3 Shifts the Equilibrium Increase concentration of product(s) Decrease concentration of product(s) Increase concentration of reactant(s) Decrease concentration of reactant(s) left right left Thus: Concentration effect the position and not the value of K
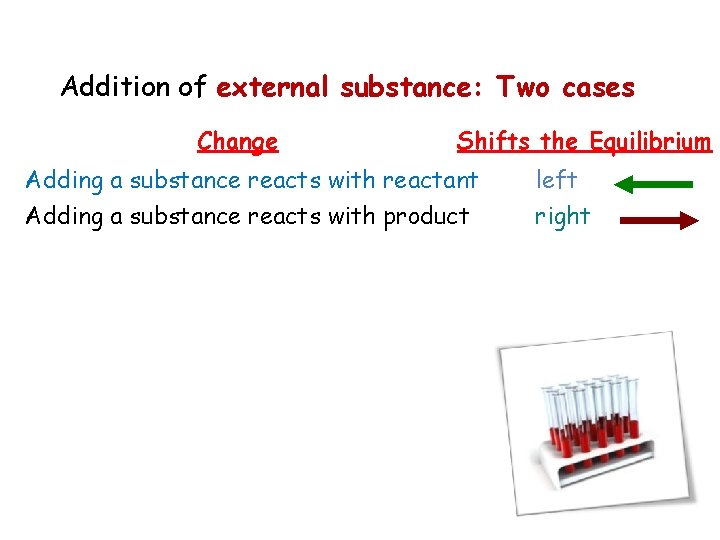
Addition of external substance: Two cases Change Shifts the Equilibrium Adding a substance reacts with reactant Adding a substance reacts with product left right
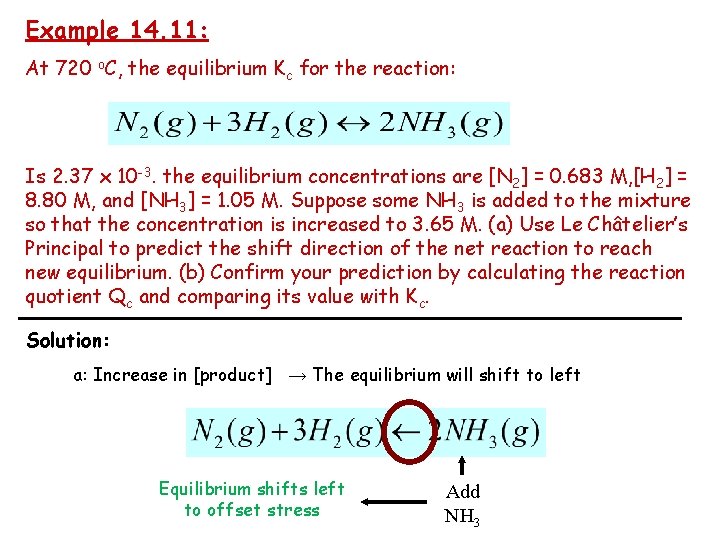
Example 14. 11: At 720 o. C, the equilibrium Kc for the reaction: Is 2. 37 x 10 -3. the equilibrium concentrations are [N 2] = 0. 683 M, [H 2] = 8. 80 M, and [NH 3] = 1. 05 M. Suppose some NH 3 is added to the mixture so that the concentration is increased to 3. 65 M. (a) Use Le Châtelier’s Principal to predict the shift direction of the net reaction to reach new equilibrium. (b) Confirm your prediction by calculating the reaction quotient Qc and comparing its value with Kc. Solution: a: Increase in [product] → The equilibrium will shift to left Equilibrium shifts left to offset stress Add NH 3
![Kc=2. 37× 10 -3 , T=720°C [N 2] = 0. 683 M [H 2] Kc=2. 37× 10 -3 , T=720°C [N 2] = 0. 683 M [H 2]](http://slidetodoc.com/presentation_image_h/dd716009c85a4b57e053f1ea96fd03e5/image-34.jpg)
Kc=2. 37× 10 -3 , T=720°C [N 2] = 0. 683 M [H 2] = 8. 80 M [NH 3] = 1. 05 M the concentration increase to 3. 65 M (b) Calculate Qc compare it value with Kc Because Qc (2. 86× 10 -2) > Kc (2. 37× 10 -3), The net reaction direction from right to left until Qc = Kc
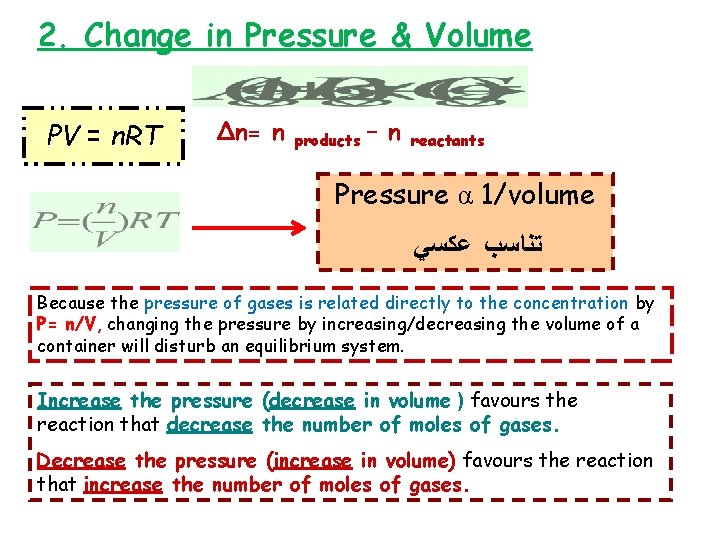
2. Change in Pressure & Volume PV = n. RT ∆n= n products – n reactants Pressure α 1/volume ﺗﻨﺎﺳﺐ ﻋﻜﺴﻲ Because the pressure of gases is related directly to the concentration by P= n/V, changing the pressure by increasing/decreasing the volume of a container will disturb an equilibrium system. Increase the pressure (decrease in volume ) favours the reaction that decrease the number of moles of gases. Decrease the pressure (increase in volume) favours the reaction that increase the number of moles of gases.
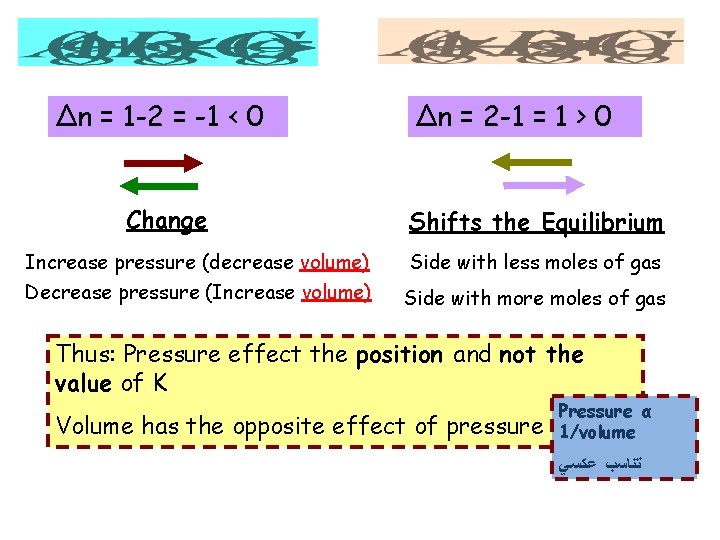
Δn = 1 -2 = -1 < 0 Change Δn = 2 -1 = 1 > 0 Shifts the Equilibrium Increase pressure (decrease volume) Side with less moles of gas Decrease pressure (Increase volume) Side with more moles of gas Thus: Pressure effect the position and not the value of K Volume has the opposite effect of pressure Pressure α 1/volume ﺗﻨﺎﺳﺐ ﻋﻜﺴﻲ
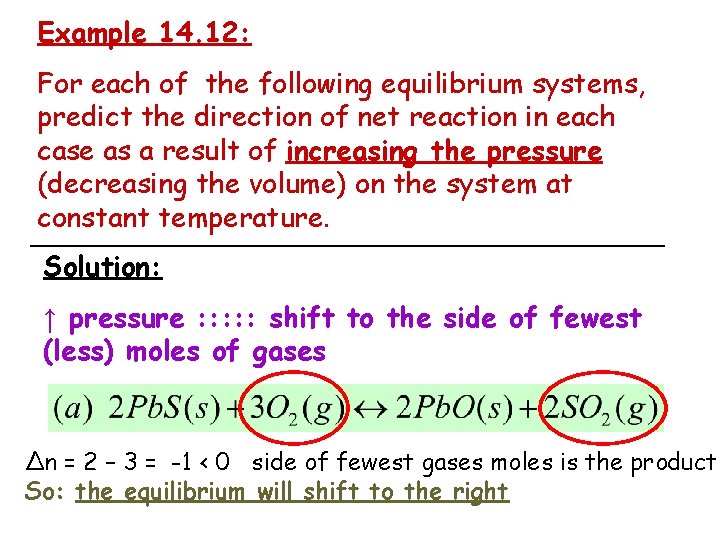
Example 14. 12: For each of the following equilibrium systems, predict the direction of net reaction in each case as a result of increasing the pressure (decreasing the volume) on the system at constant temperature. Solution: ↑ pressure : : : shift to the side of fewest (less) moles of gases Δn = 2 – 3 = -1 < 0 side of fewest gases moles is the product So: the equilibrium will shift to the right
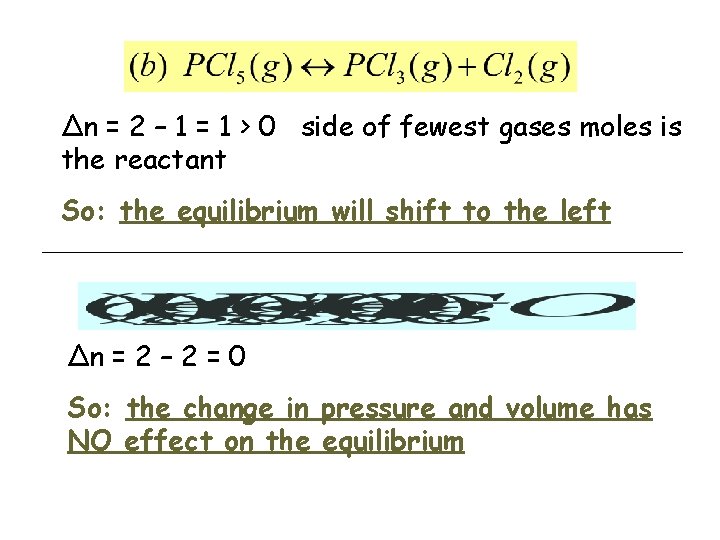
Δn = 2 – 1 = 1 > 0 side of fewest gases moles is the reactant So: the equilibrium will shift to the left Δn = 2 – 2 = 0 So: the change in pressure and volume has NO effect on the equilibrium
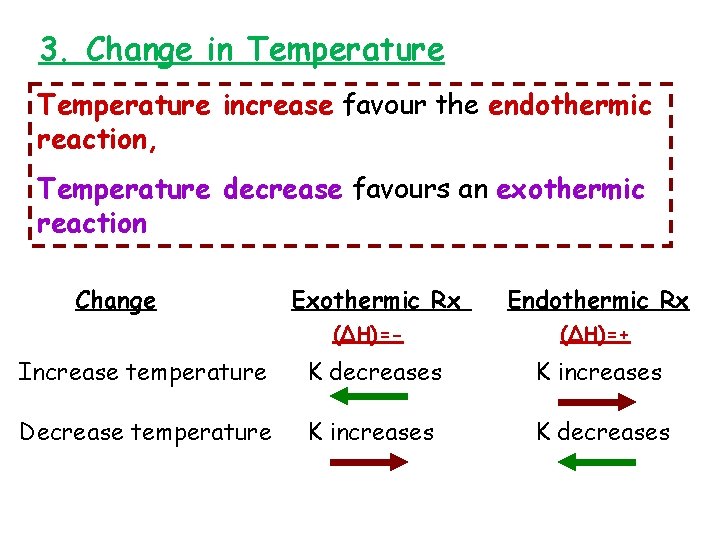
3. Change in Temperature increase favour the endothermic reaction, Temperature decrease favours an exothermic reaction Change Exothermic Rx (∆H)=- Endothermic Rx (∆H)=+ Increase temperature K decreases K increases Decrease temperature K increases K decreases
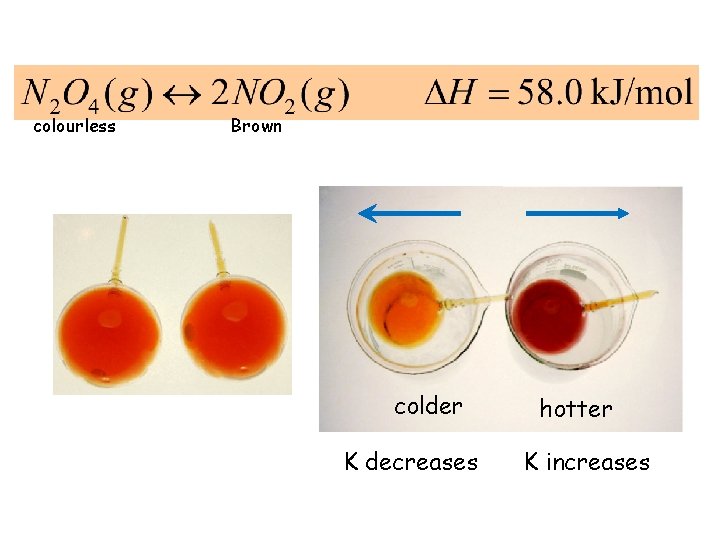
colourless Brown colder K decreases hotter K increases
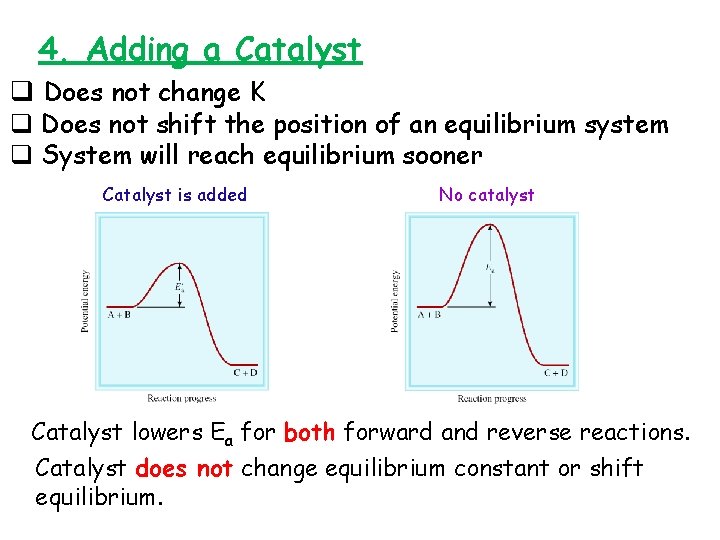
4. Adding a Catalyst q Does not change K q Does not shift the position of an equilibrium system q System will reach equilibrium sooner Catalyst is added No catalyst Catalyst lowers Ea for both forward and reverse reactions. Catalyst does not change equilibrium constant or shift equilibrium.
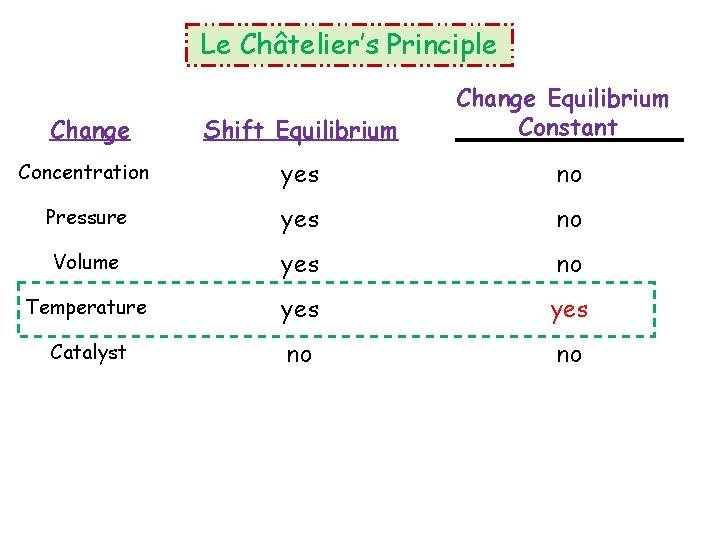
Le Châtelier’s Principle Shift Equilibrium Change Equilibrium Constant Concentration yes no Pressure yes no Volume yes no Temperature yes Catalyst no no Change

Example 14. 13: Predict the net reaction direction a) if RXN heated at constant V, b) some N 2 F 4 removed at constant T&V c) Decrease P? d) catalyst is added. q a) ∆H > 0 >>endothermic reaction , T increase, K decreases, a net change occurs in the direction is from left to right toward product). q b) Conc. of the reactant decrease the system shift to left ( some NF 2 combines to produce N 2 F 4 ) q c) P decrease the system shift to right. q d) if catalyst is added to reaction mixture , the reaction will reach equilibrium faster but no change in the change equilibrium constant or shift equilibrium.

Problems 14. 3, 14. 8, 14. 16, 14. 18, 14. 22, 14. 48, 14. 72, 14. 51, 14. 54, 14. 56, 14. 58, 14. 62
- Slides: 44