Chemical Equilibrium Chapter 13 Equilibrium is a state

Chemical Equilibrium Chapter 13
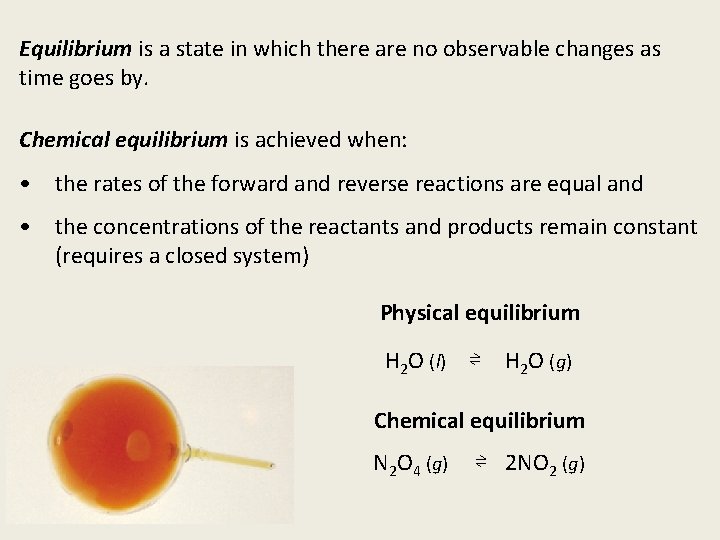
Equilibrium is a state in which there are no observable changes as time goes by. Chemical equilibrium is achieved when: • the rates of the forward and reverse reactions are equal and • the concentrations of the reactants and products remain constant (requires a closed system) Physical equilibrium H 2 O (l) ⇌ H 2 O (g) Chemical equilibrium N 2 O 4 (g) ⇌ 2 NO 2 (g)

Characteristics of a system at equilibrium 1. Reversible reaction must be possible 2. There is a dynamic state of balance between both the forward and backward reaction 3. There is no change in concentration of reactants or products once chemical equilibrium is reached 4. There is no bulk change in properties of the system (ex: no colour or pressure change)

5. It is a closed system (no heat or matter in or out) 6. The temperature of the system remains constant 7. It can be reached from either direction 8. Any change to the system at equilibrium can be reversed and restored back to original equilibrium conditions.

Examples at Equilibrium • Water evaporating and condensing in a jar with lid • A saturated solution with a few crystals added • A bottle of unopened cola, carbon dioxide is in equilibrium in solution and air above pop.

Writing equilibrium expressions – keq expressions See rules pg. 27 -28 Hebden 1. All gases must be included. 2. Aqueous ions must be included. 3. Solids are NEVER included. 4. Pure liquids are NEVER included. 5. Mixtures of liquids must be included. Assign Hebden pg. 28 #1 -10


• Changing the temperature affects the rates of forward and reverse reactions differently because they have different activation energies. • ie. Changing the temperature will change the equilibrium and establish a NEW and DIFFERENT equilibrium. All other changes maintain the same equilibrium.
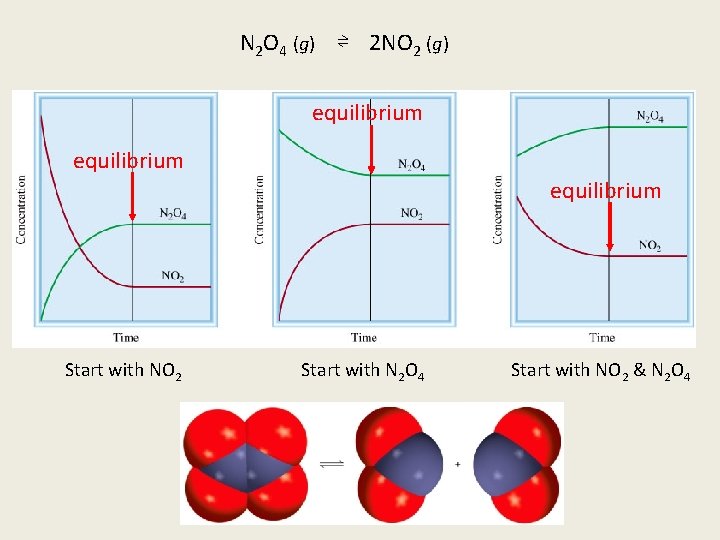
N 2 O 4 (g) ⇌ 2 NO 2 (g) equilibrium Start with NO 2 Start with N 2 O 4 Start with NO 2 & N 2 O 4
![K= [C]c[D]d a. A + b. B ⇌ c. C + d. D [A]a[B]b K= [C]c[D]d a. A + b. B ⇌ c. C + d. D [A]a[B]b](http://slidetodoc.com/presentation_image_h2/54e739dcb50738396ea8b2693e7e11c6/image-10.jpg)
K= [C]c[D]d a. A + b. B ⇌ c. C + d. D [A]a[B]b Equilibrium Will K >> 1 Lie to the right Favor products K << 1 Lie to the left Favor reactants
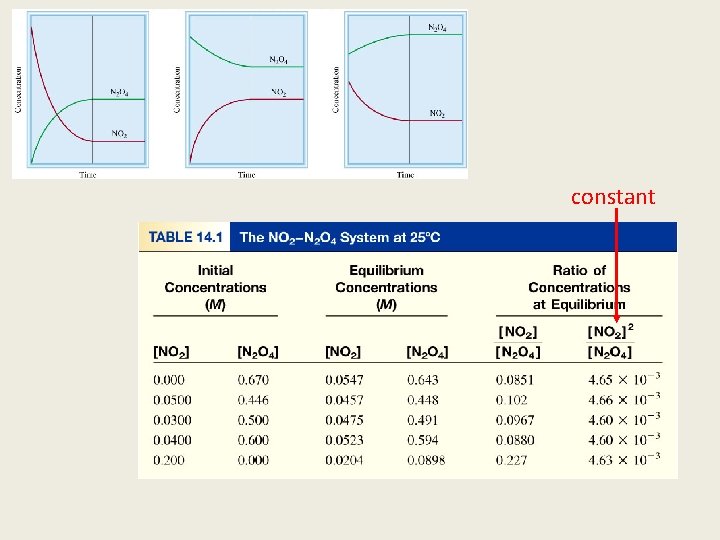
constant
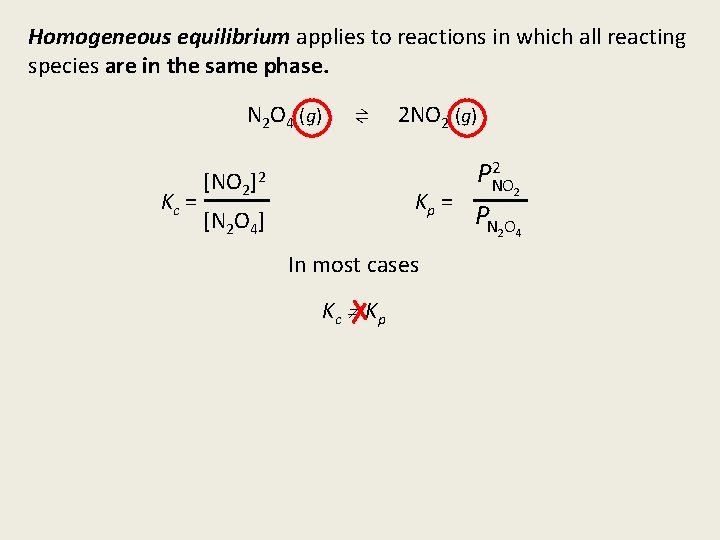
Homogeneous equilibrium applies to reactions in which all reacting species are in the same phase. N 2 O 4 (g) Kc = [NO 2 ⇌ ]2 2 NO 2 (g) Kp = [N 2 O 4] In most cases Kc Kp 2 PNO 2 PN 2 O 4
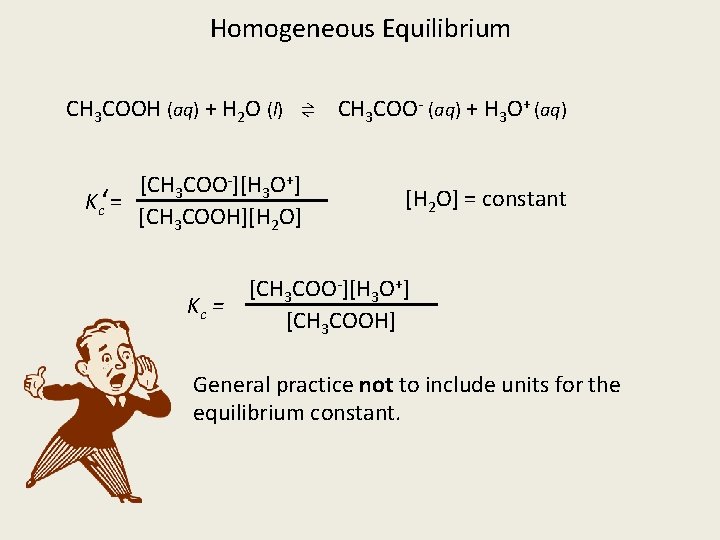
Homogeneous Equilibrium CH 3 COOH (aq) + H 2 O (l) ⇌ CH 3 COO- (aq) + H 3 O+ (aq) [CH 3 COO-][H 3 O+] Kc‘= [CH 3 COOH][H 2 O] Kc = [H 2 O] = constant [CH 3 COO-][H 3 O+] [CH 3 COOH] General practice not to include units for the equilibrium constant.
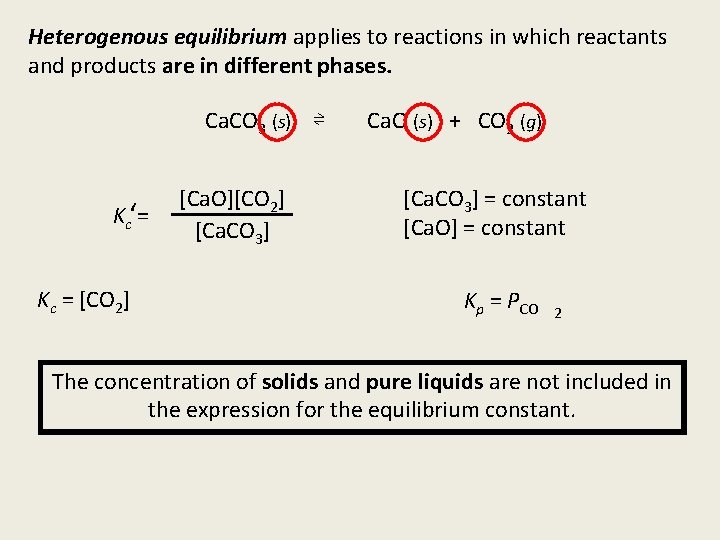
Heterogenous equilibrium applies to reactions in which reactants and products are in different phases. Ca. CO 3 (s) ⇌ Kc‘= Kc = [CO 2] [Ca. O][CO 2] [Ca. CO 3] Ca. O (s) + CO 2 (g) [Ca. CO 3] = constant [Ca. O] = constant Kp = PCO 2 The concentration of solids and pure liquids are not included in the expression for the equilibrium constant.
![N 2 O 4 (g) ⇌ 2 NO 2 (g) K= [NO 2]2 [N N 2 O 4 (g) ⇌ 2 NO 2 (g) K= [NO 2]2 [N](http://slidetodoc.com/presentation_image_h2/54e739dcb50738396ea8b2693e7e11c6/image-15.jpg)
N 2 O 4 (g) ⇌ 2 NO 2 (g) K= [NO 2]2 [N 2 O 4] = 4. 63 x 10 -3 2 NO 2 (g) ⇌ N 2 O 4 (g) [N 2 O 4] 1 = 216 = K ‘= 2 K [NO 2] When the equation for a reversible reaction is written in the opposite direction, the equilibrium constant becomes the reciprocal of the original equilibrium constant.
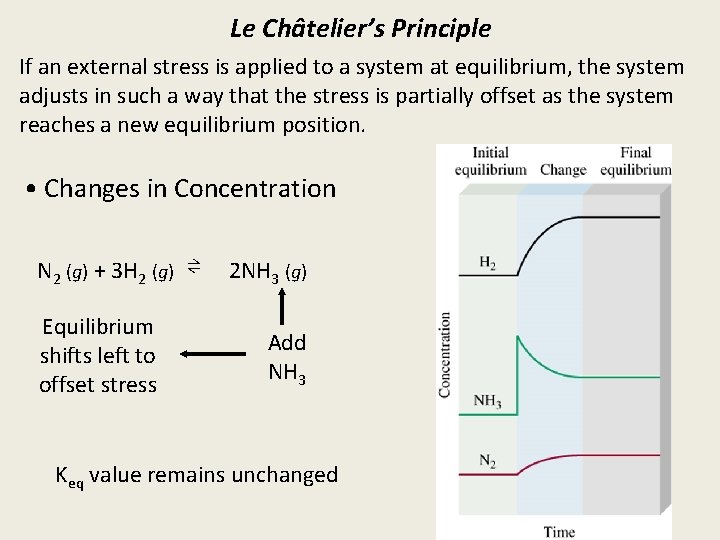
Le Châtelier’s Principle If an external stress is applied to a system at equilibrium, the system adjusts in such a way that the stress is partially offset as the system reaches a new equilibrium position. • Changes in Concentration N 2 (g) + 3 H 2 (g) ⇌ Equilibrium shifts left to offset stress 2 NH 3 (g) Add NH 3 Keq value remains unchanged
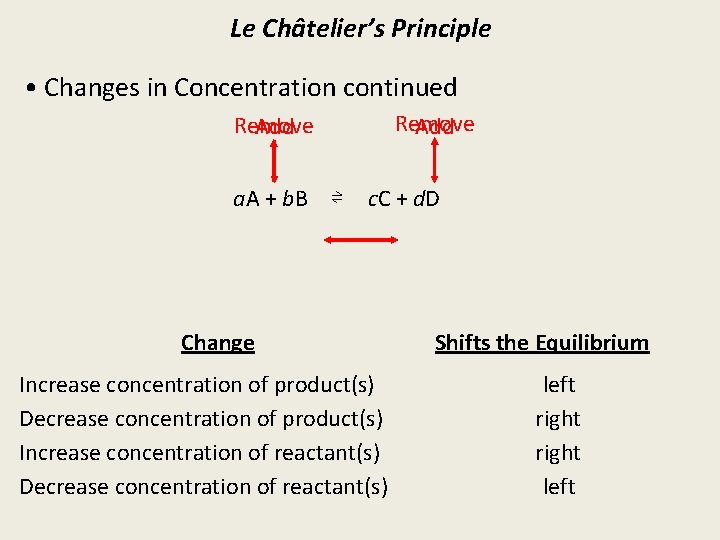
Le Châtelier’s Principle • Changes in Concentration continued Remove Add a. A + b. B ⇌ c. C + d. D Change Increase concentration of product(s) Decrease concentration of product(s) Increase concentration of reactant(s) Decrease concentration of reactant(s) Shifts the Equilibrium left right left
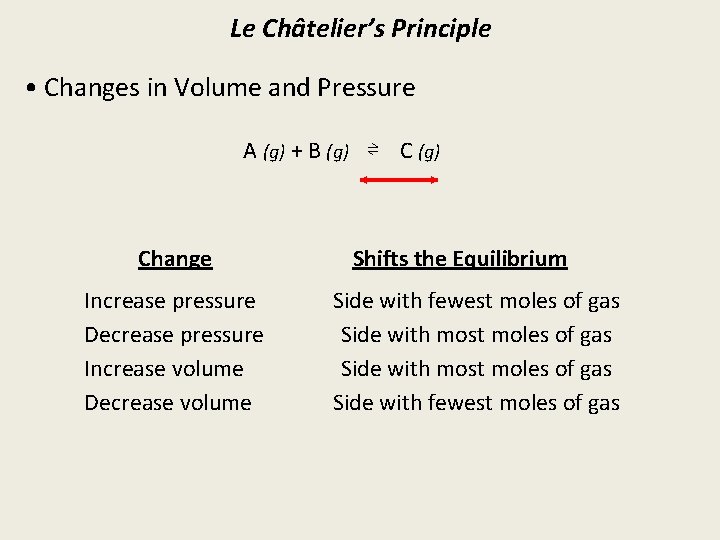
Le Châtelier’s Principle • Changes in Volume and Pressure A (g) + B (g) ⇌ C (g) Change Increase pressure Decrease pressure Increase volume Decrease volume Shifts the Equilibrium Side with fewest moles of gas Side with most moles of gas Side with fewest moles of gas
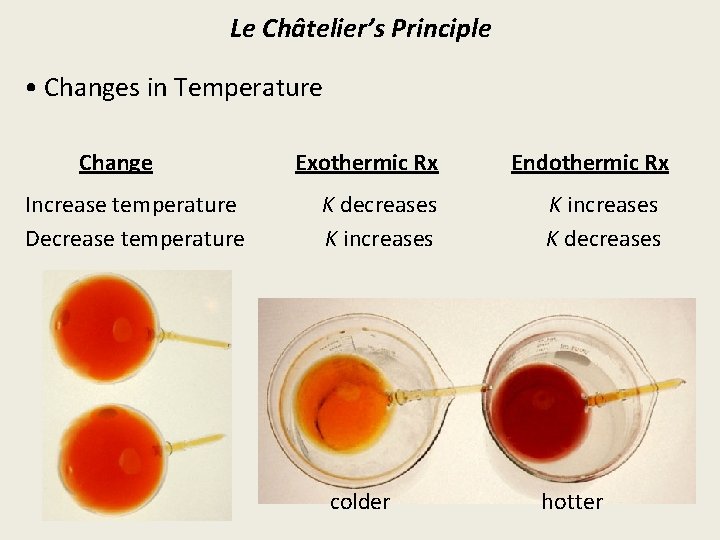
Le Châtelier’s Principle • Changes in Temperature Change Increase temperature Decrease temperature Exothermic Rx K decreases K increases colder Endothermic Rx K increases K decreases hotter

• Changing the temperature affects the rates of forward and reverse reactions differently because they have different activation energies
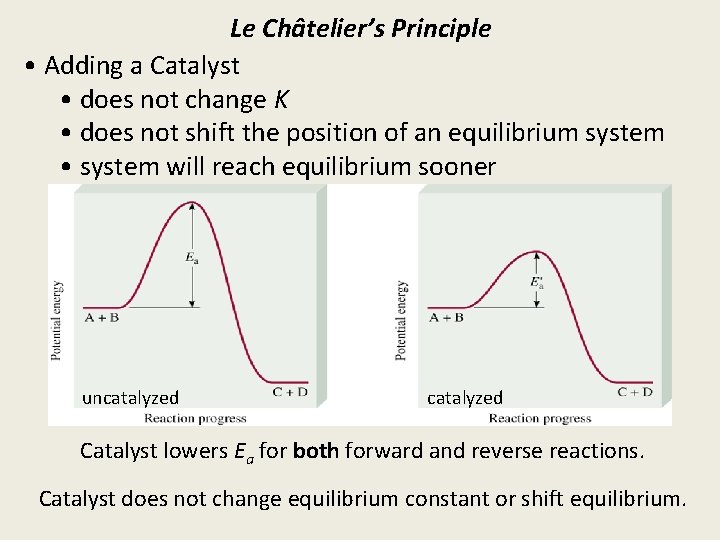
Le Châtelier’s Principle • Adding a Catalyst • does not change K • does not shift the position of an equilibrium system • system will reach equilibrium sooner uncatalyzed Catalyst lowers Ea for both forward and reverse reactions. Catalyst does not change equilibrium constant or shift equilibrium.
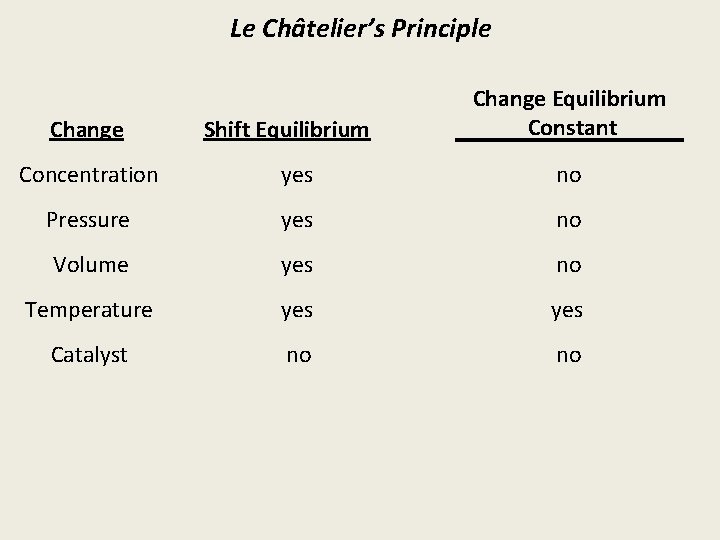
Le Châtelier’s Principle Change Shift Equilibrium Change Equilibrium Constant Concentration yes no Pressure yes no Volume yes no Temperature yes Catalyst no no
- Slides: 22