Chemical Equations Reactions Indications of a Chemical Reaction


















- Slides: 25

Chemical Equations & Reactions

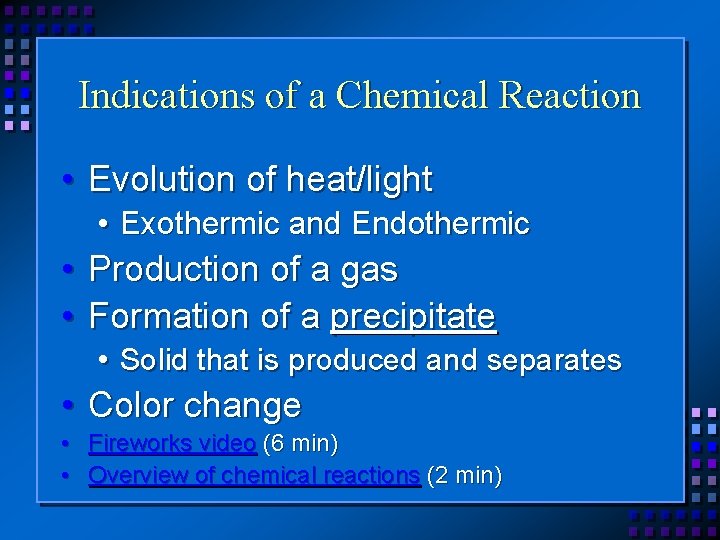
Indications of a Chemical Reaction • Evolution of heat/light • Exothermic and Endothermic • Production of a gas • Formation of a precipitate • Solid that is produced and separates • Color change • Fireworks video (6 min) • Overview of chemical reactions (2 min)

Factors Influencing Rate of Reaction • • • Nature of reactants Surface area Temperature Concentration Catalyst • Increased surface area

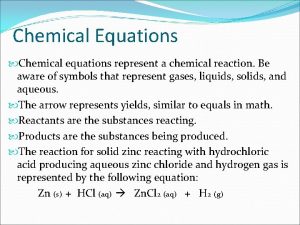
Chemical Equations • Chemical Equation = represents, with symbols and formulas, the identities and relative amounts of reactants and products • Reactants (R) – left of arrow • Products (P) – right of arrow

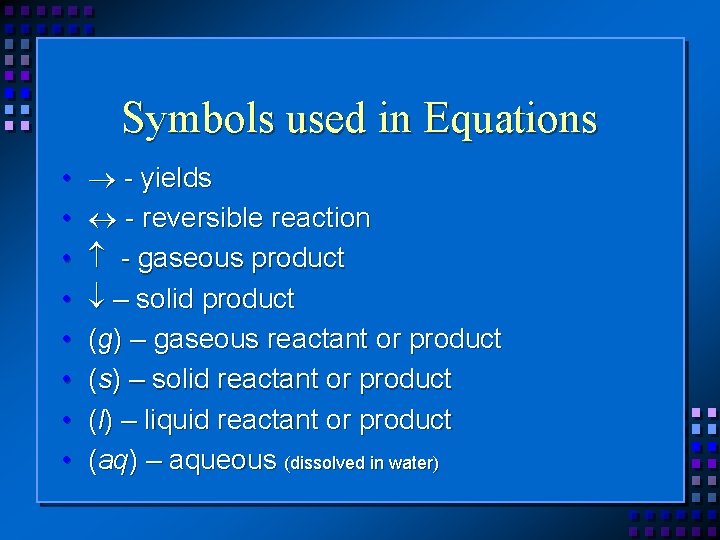
Symbols used in Equations • • - yields - reversible reaction - gaseous product – solid product (g) – gaseous reactant or product (s) – solid reactant or product (l) – liquid reactant or product (aq) – aqueous (dissolved in water)

Writing Chemical Equations 1. Must have correct formulas for each R and P • Don’t forget diatomic elements Br 2, I 2, N 2, Cl 2, H 2, O 2, F 2 2. Law of Conservation of Mass must be satisfied • Balance the equation with coefficients

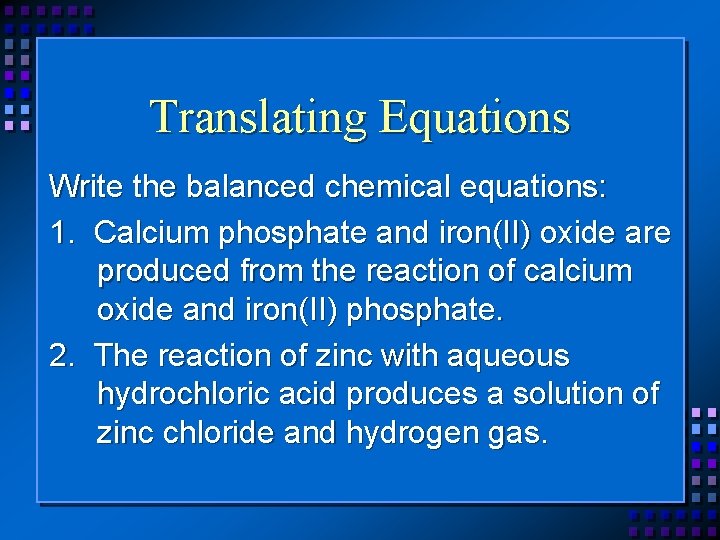
Translating Equations Write the balanced chemical equations: 1. Calcium phosphate and iron(II) oxide are produced from the reaction of calcium oxide and iron(II) phosphate. 2. The reaction of zinc with aqueous hydrochloric acid produces a solution of zinc chloride and hydrogen gas.

Types of Chemical Reactions • • • Synthesis Decomposition Single Replacement Double Replacement Combustion

Synthesis Reaction • Two or more substances combine to form one product • EX: Calcium + oxygen → ? ? • EX: Barium + chlorine → ? ?

Decomposition Reaction • Single compound breaks down into several simpler substances • There are 4 types of decomposition reactions

Binary Decomposition • Metal oxide → metal + oxygen gas • EX: Zinc oxide → ? ? • Decomposition of nitrogen triiodide

Metal Carbonate Decomposition • Metal carbonate → metal oxide + carbon dioxide • EX: Strontium carbonate → ? ?

Metal Chlorate Decomposition • Metal chlorate → metal chloride and oxygen gas • EX: Iron (III) chlorate → ? ? • EX: Potassium hypochlorite → ? ?

Metal Hydroxide Decomposition • Metal hydroxide → metal oxide + water • EX: Calcium hydroxide → ? ?

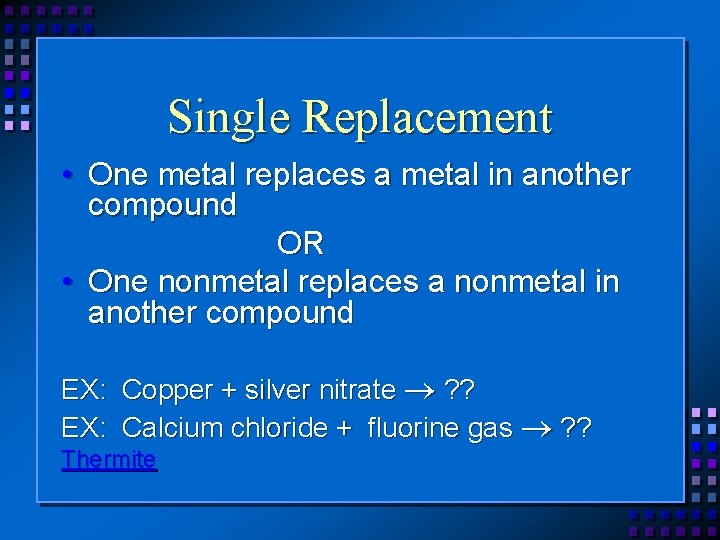
Single Replacement • One metal replaces a metal in another compound OR • One nonmetal replaces a nonmetal in another compound EX: Copper + silver nitrate ? ? EX: Calcium chloride + fluorine gas ? ? Thermite

Will a SR reaction always occur? ? • Check the Activity Series • The more reactive element will make a bond. If the more reactive element is already bonded, write NR for no reaction. • EX: Silver + copper (I) nitrate ? ? • Reactivity of Alkali Metals

Double Replacement • Ions of 2 reactants exchange places in an aqueous solution to form new products • EX: Zinc oxalate + ammonium phosphate → ? ?

More on Double Replacement • Some of the products may exist as ions in an aqueous solution, while others may exist as a solid • Soluble Cmpd (aq) = Exists as ions in solution • Insoluble Cmpd (s) = Exists as a solid in solution

Soluble or Insoluble? ? • Check the solubility chart or rules on reference sheet • Be sure to indicate which product is soluble and which is insoluble!!!!! • Double Replacement - Production of Precipitate

Examples of Solubility • Are these compounds soluble or insoluble? 1. K 2 SO 4 2. Ag. Cl 3. Ba. SO 4 4. Ca. S 5. K 3 PO 4 6. Mg. CO 3

Net Ionic Equations • Includes only the compounds and ions that are chemically changed • Step 1: Write the products in words. Translate into a balanced equation – including states (use solubility rules) • Step 2: Write the overall ionic equation – cancel spectator ions. • Step 3: Write the net ionic equation – including states

Example Zinc nitrate + ammonium sulfide → ? ?

Combustion • A substance reacts with oxygen to produce lots of energy, usually in the form of heat and light

Complete Combustion • C_H_ + O 2 H 2 O + CO 2 + energy • EX: Propane + oxygen gas → ? ? • • • Combustion Video (5 min) Origin of Combustion Engine Part 1(2 min) Origin of Combustion Engine Part 2 (3 min) Hindenburg Disaster (4 min)

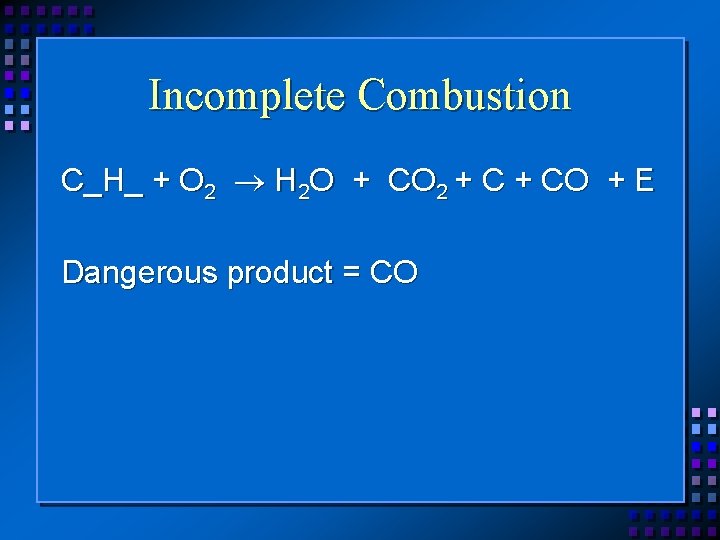
Incomplete Combustion C_H_ + O 2 H 2 O + CO 2 + CO + E Dangerous product = CO
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Are kc and kp equal
Are kc and kp equal Indications of chemical reaction
Indications of chemical reaction Indications of chemical reactions
Indications of chemical reactions Types of reactions
Types of reactions Lesson 68 toxic reactions chemical equations
Lesson 68 toxic reactions chemical equations Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Balancing chemical equations definition
Balancing chemical equations definition Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Toxic reactions chemical equations
Toxic reactions chemical equations Section 1 chemical changes
Section 1 chemical changes Difference between nuclear reaction and chemical reaction
Difference between nuclear reaction and chemical reaction Half redox reaction
Half redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Translating chemical equations
Translating chemical equations Stoichiometry mole island diagram
Stoichiometry mole island diagram Types of chemical reactions redox
Types of chemical reactions redox Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Types of chemical reactions
Types of chemical reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions