Chemical equations Reactions Chemical equations What is a



- Slides: 9

Chemical equations Reactions!!!!

Chemical equations § What is a chemical symbol? § A symbol is a 1 -3 letter representation of an atom of a single element. § How is it written? § The first letter is capitalized, the following letters are lower case (if they are there) §C § Na § Uun

Chemical equations § What is a chemical formula? § A formula is a representation of a compound (bonds) using chemical symbols. § How is a formula written? § Each symbol represents one atom, and each subscript multiplies the atoms by that number. § Na. Cl § H 2 O

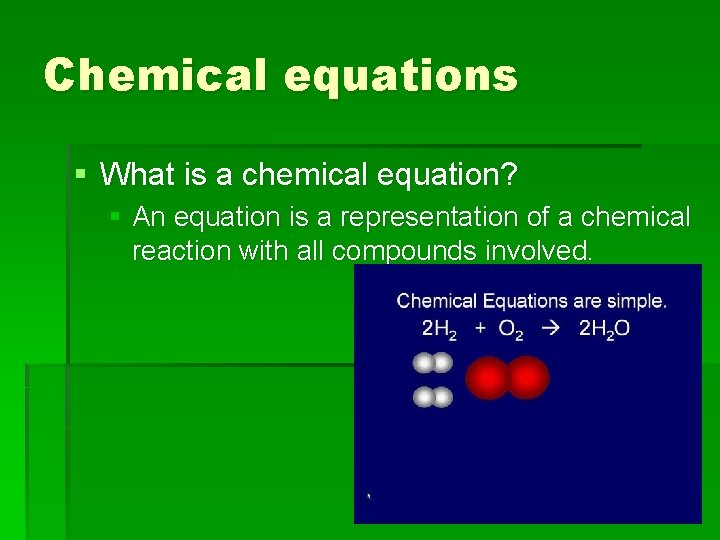
Chemical equations § What is a chemical equation? § An equation is a representation of a chemical reaction with all compounds involved.

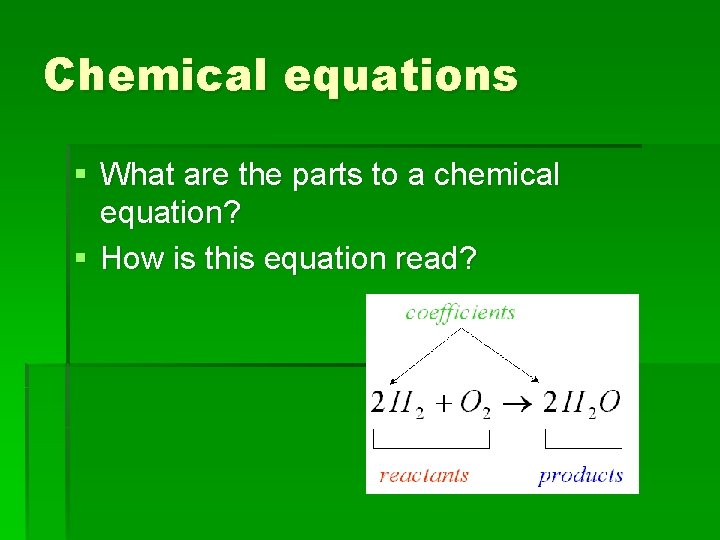
Chemical equations § What are the parts to a chemical equation? § How is this equation read?

Chemical equations § What is the law of conservation of mass? § This law states that under ordinary circumstances, matter can be neither created nor destroyed. § What does this mean for chemical equations?

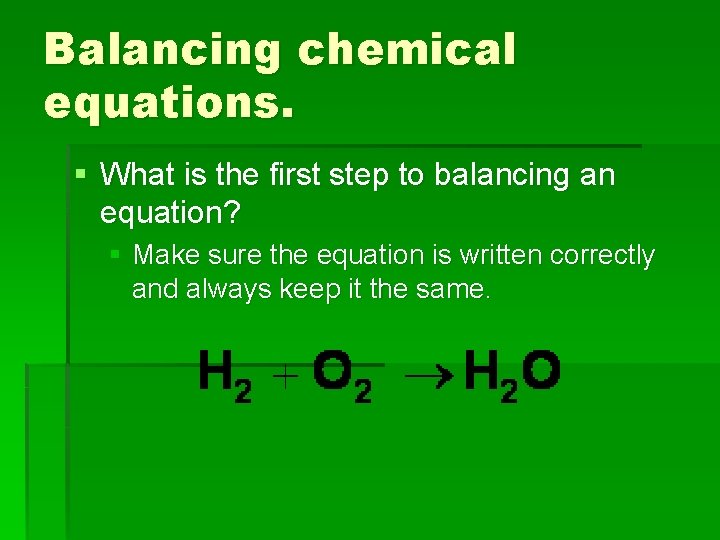
Balancing chemical equations. § What is the first step to balancing an equation? § Make sure the equation is written correctly and always keep it the same.

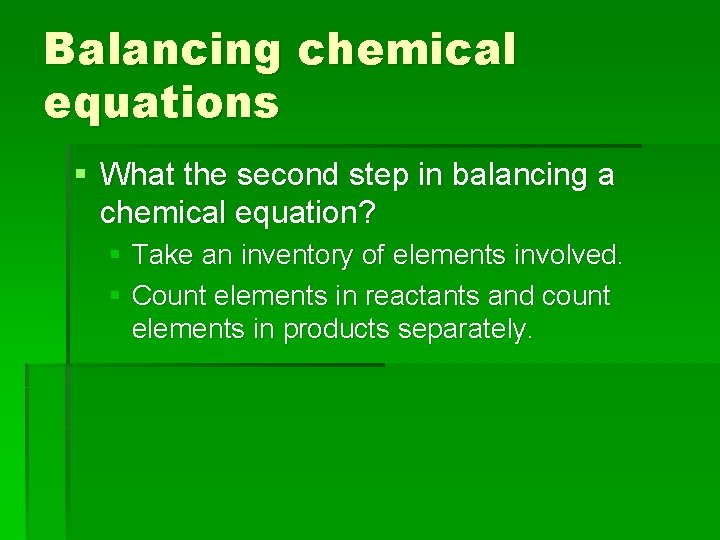
Balancing chemical equations § What the second step in balancing a chemical equation? § Take an inventory of elements involved. § Count elements in reactants and count elements in products separately.

Balancing chemical equations § What is the final step in balancing a chemical equation? § Use coefficients to make the number of elements on each side equal. § You cannot change a chemical formula.
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Types of reactions
Types of reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Unit 4: toxins lesson 73 worksheet answers
Unit 4: toxins lesson 73 worksheet answers Chemistry chapter 8 review chemical equations and reactions
Chemistry chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Unit 5 chemical reactions
Unit 5 chemical reactions

















