Chemical Equations Reactions Chapter 8 Objectives l l
Chemical Equations & Reactions Chapter 8

Objectives l l List observations that suggest that a chemical reaction has taken place. List three requirements for a correctly written chemical equation. Write a word equation and a formula equation for a given chemical reaction. Balance a formula equation by inspection.

Chemical Reaction Process by which one or more substances are changed into one or more different substances
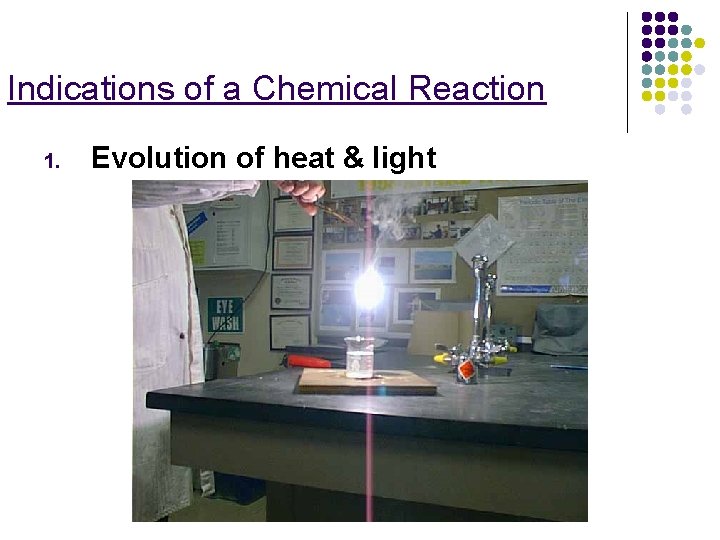
Indications of a Chemical Reaction 1. Evolution of heat & light

Indications of a Chemical Reaction 2. Production of a gas
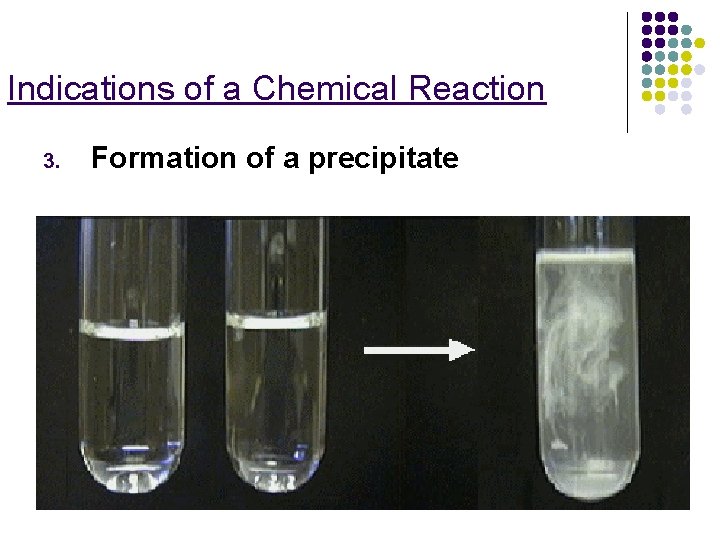
Indications of a Chemical Reaction 3. Formation of a precipitate

Precipitate A solid that is produced as a result of a chemical reaction in solution & that separates from the solution

Indications of a chemical reaction 4. Change in color. (sumac leaves change color when chlorophyll is destroyed)
Chemical Equation Representation, with symbols and formulas, of the identities and relative amounts of the reactants and products in a chemical reaction Reactants Products Follows the Law of Conservation of Mass
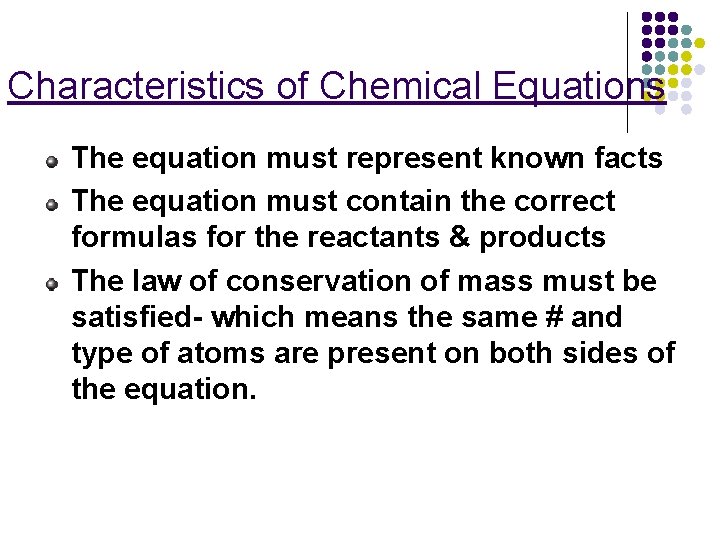
Characteristics of Chemical Equations The equation must represent known facts The equation must contain the correct formulas for the reactants & products The law of conservation of mass must be satisfied- which means the same # and type of atoms are present on both sides of the equation.

Writing Chemical Equations Word Equation Represents facts- only qualitative Example: Methane + oxygen carbon dioxide + water
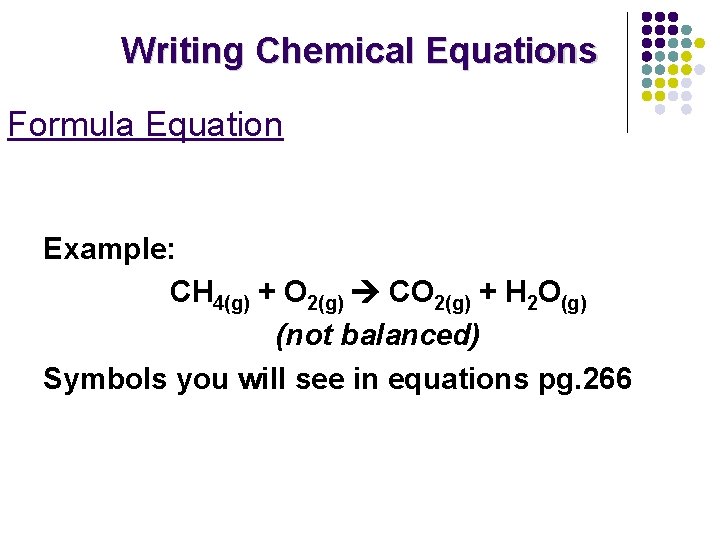
Writing Chemical Equations Formula Equation Example: CH 4(g) + O 2(g) CO 2(g) + H 2 O(g) (not balanced) Symbols you will see in equations pg. 266
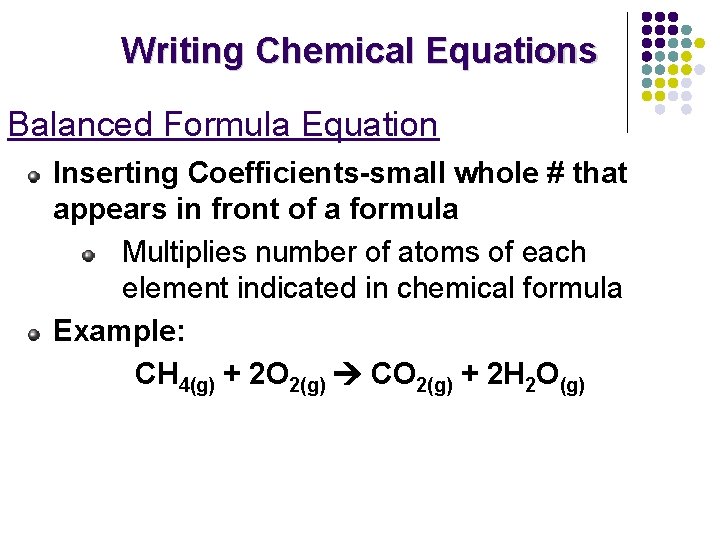
Writing Chemical Equations Balanced Formula Equation Inserting Coefficients-small whole # that appears in front of a formula Multiplies number of atoms of each element indicated in chemical formula Example: CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(g)
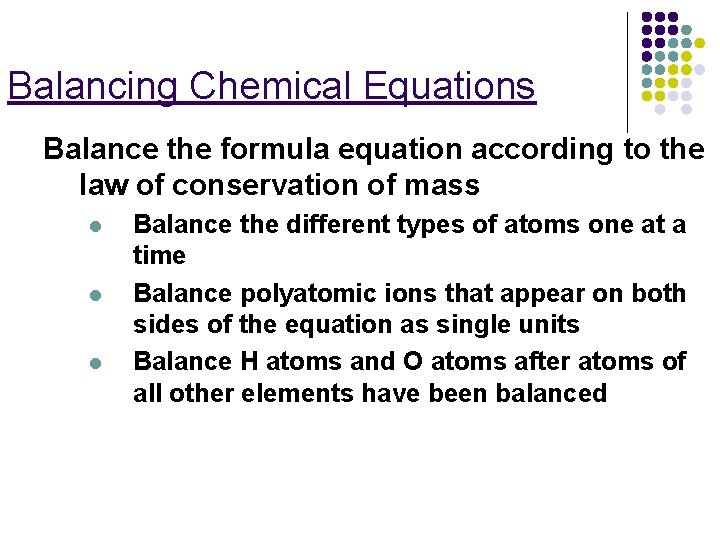
Balancing Chemical Equations Balance the formula equation according to the law of conservation of mass l l l Balance the different types of atoms one at a time Balance polyatomic ions that appear on both sides of the equation as single units Balance H atoms and O atoms after atoms of all other elements have been balanced
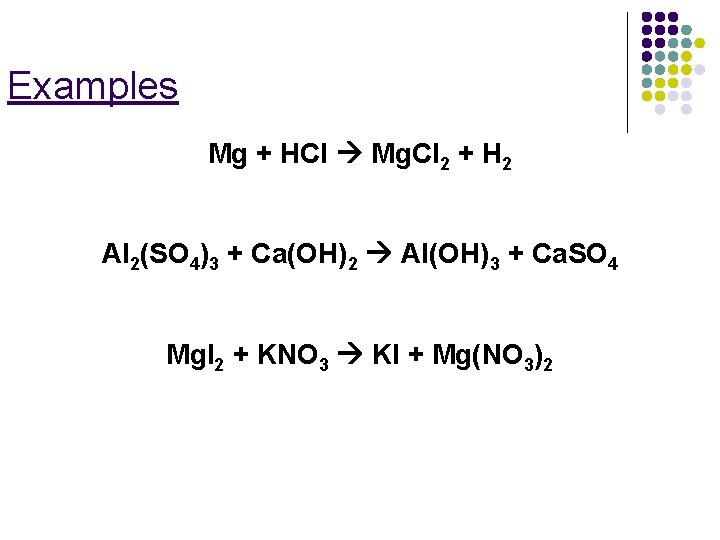
Examples Mg + HCl Mg. Cl 2 + H 2 Al 2(SO 4)3 + Ca(OH)2 Al(OH)3 + Ca. SO 4 Mg. I 2 + KNO 3 KI + Mg(NO 3)2

Writing and Balancing Equations from Word Equations 1. Write out correct chemical formulas and symbols. 2. Balance equation. Solid aluminum metal combines with oxygen gas to produce solid aluminum oxide

Writing equations from word equations Examples to try. l 1. Solid calcium metal reacts with water to form aqueous calcium hydroxide and hydrogen gas. 2. Solid iron(III)oxide and carbon monoxide gas produces solid iron and carbon dioxide gas. 3. Solid copper reacts with aqueous silver nitrate to produce aqueous copper (II) nitrate and solid silver.

Objectives l l l Define & give general equations for synthesis, decomposition, singlereplacement, and double-replacement reactions. Classify a reaction as synthesis, decomposition, single displacement, double displacement or combustion. Predict the products of simple reactions if given the reactants.
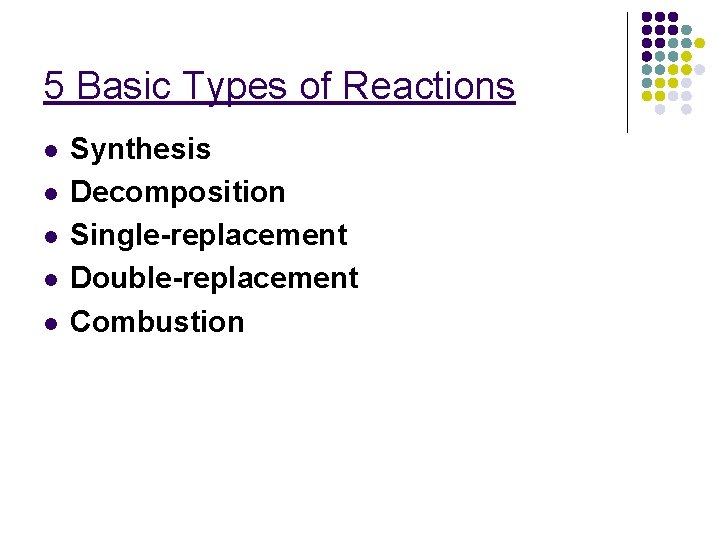
5 Basic Types of Reactions l l l Synthesis Decomposition Single-replacement Double-replacement Combustion
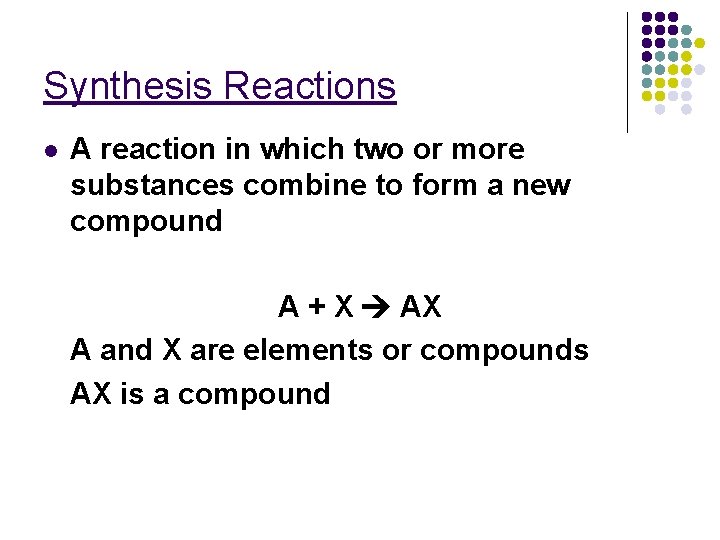
Synthesis Reactions l A reaction in which two or more substances combine to form a new compound A + X AX A and X are elements or compounds AX is a compound
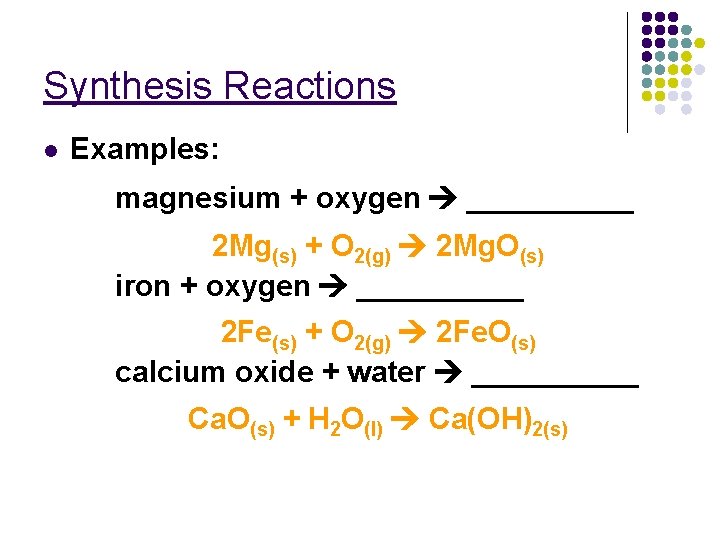
Synthesis Reactions l Examples: magnesium + oxygen _____ 2 Mg(s) + O 2(g) 2 Mg. O(s) iron + oxygen _____ 2 Fe(s) + O 2(g) 2 Fe. O(s) calcium oxide + water _____ Ca. O(s) + H 2 O(l) Ca(OH)2(s)
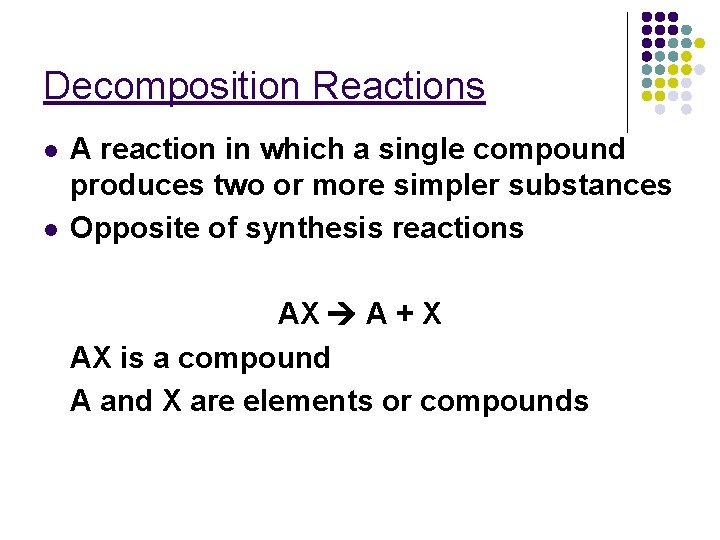
Decomposition Reactions l l A reaction in which a single compound produces two or more simpler substances Opposite of synthesis reactions AX A + X AX is a compound A and X are elements or compounds
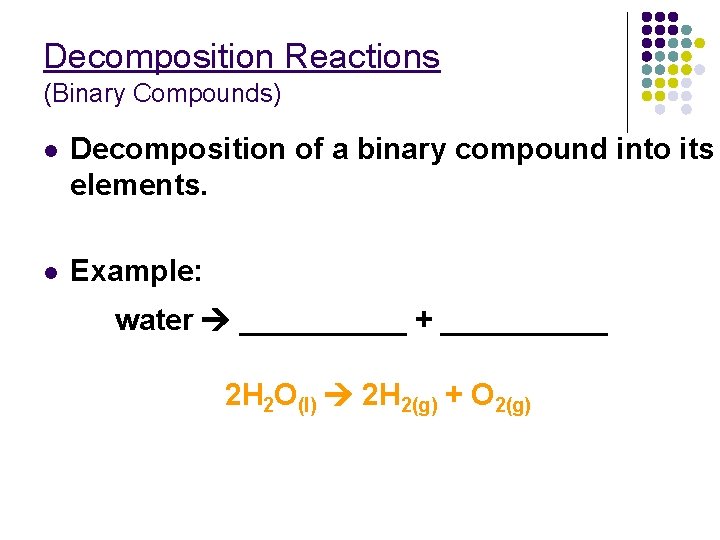
Decomposition Reactions (Binary Compounds) l Decomposition of a binary compound into its elements. l Example: water _____ + _____ 2 H 2 O(l) 2 H 2(g) + O 2(g)

Electrolysis The decomposition of a substance by an electric current
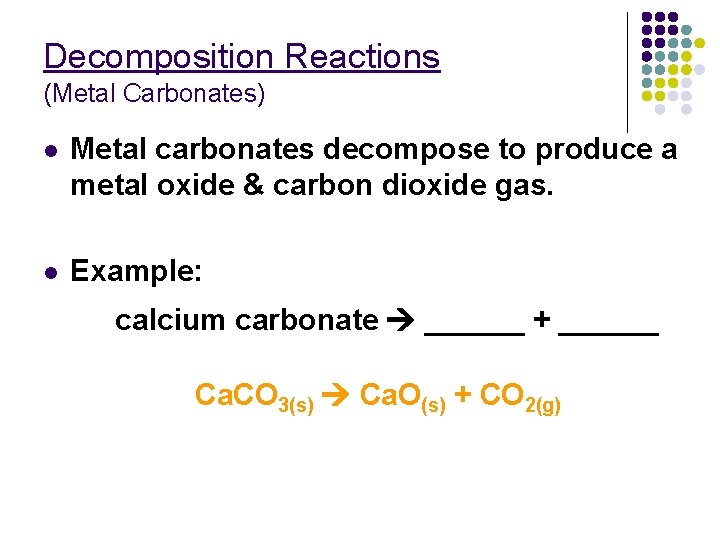
Decomposition Reactions (Metal Carbonates) l Metal carbonates decompose to produce a metal oxide & carbon dioxide gas. l Example: calcium carbonate ______ + ______ Ca. CO 3(s) Ca. O(s) + CO 2(g)
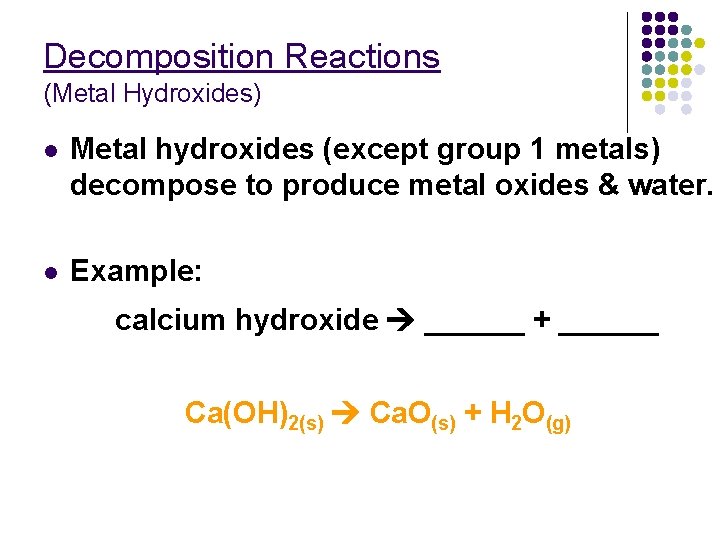
Decomposition Reactions (Metal Hydroxides) l Metal hydroxides (except group 1 metals) decompose to produce metal oxides & water. l Example: calcium hydroxide ______ + ______ Ca(OH)2(s) Ca. O(s) + H 2 O(g)
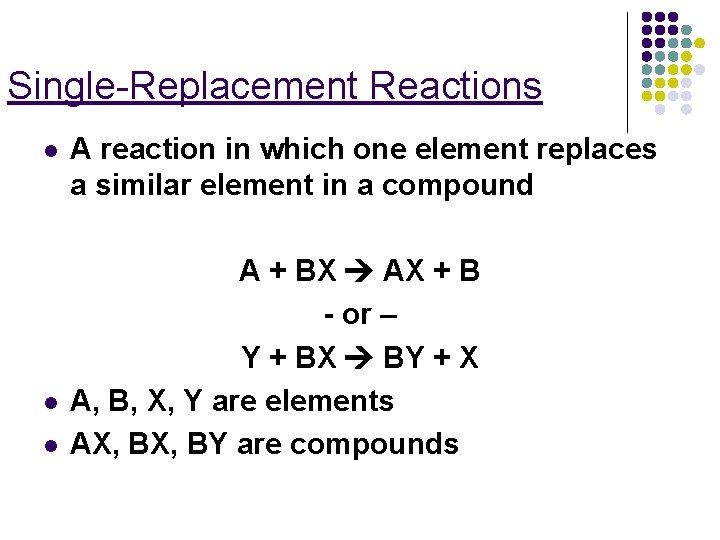
Single-Replacement Reactions l l l A reaction in which one element replaces a similar element in a compound A + BX AX + B - or – Y + BX BY + X A, B, X, Y are elements AX, BY are compounds
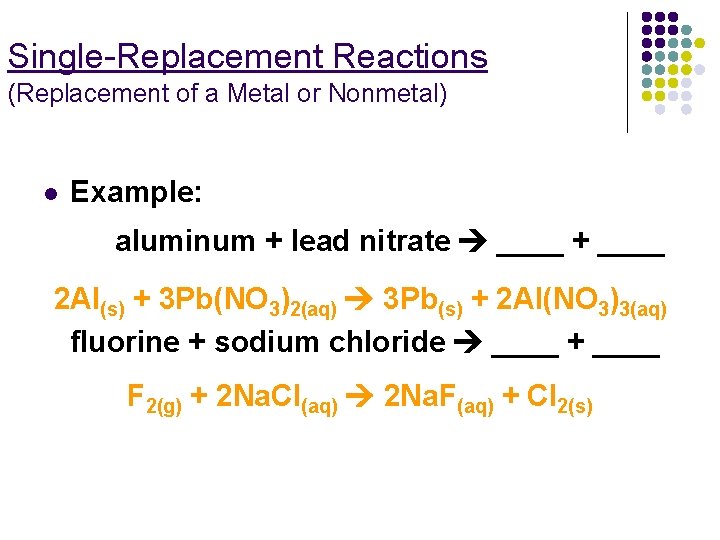
Single-Replacement Reactions (Replacement of a Metal or Nonmetal) l Example: aluminum + lead nitrate ____ + ____ 2 Al(s) + 3 Pb(NO 3)2(aq) 3 Pb(s) + 2 Al(NO 3)3(aq) fluorine + sodium chloride ____ + ____ F 2(g) + 2 Na. Cl(aq) 2 Na. F(aq) + Cl 2(s)
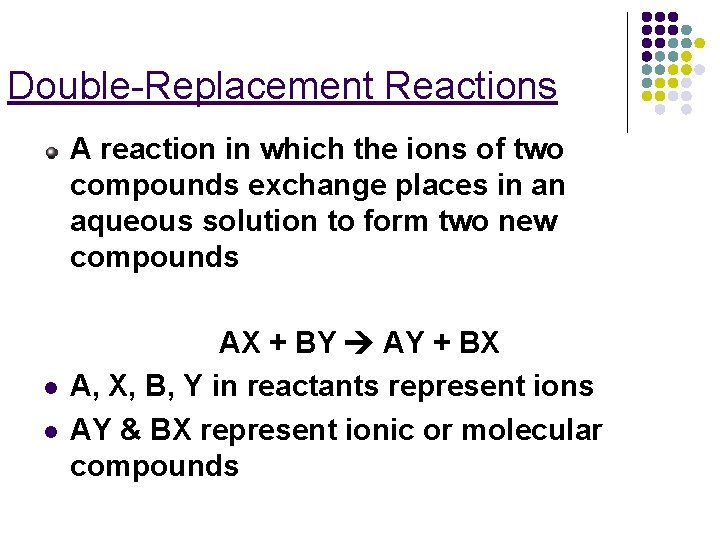
Double-Replacement Reactions A reaction in which the ions of two compounds exchange places in an aqueous solution to form two new compounds l l AX + BY AY + BX A, X, B, Y in reactants represent ions AY & BX represent ionic or molecular compounds
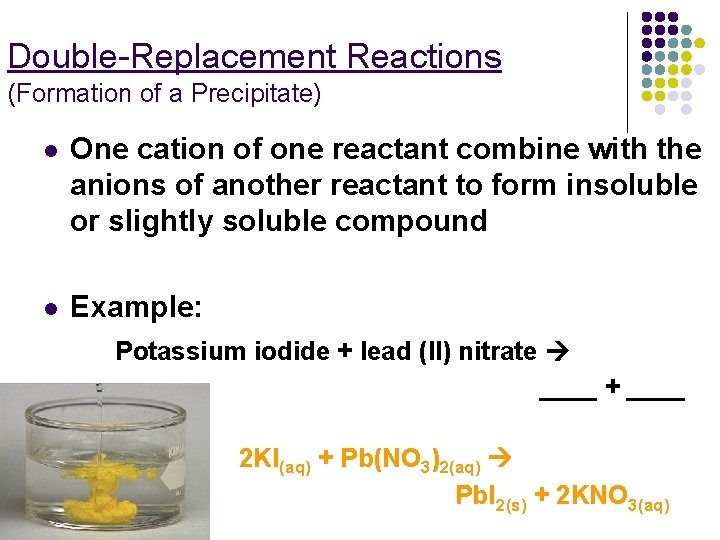
Double-Replacement Reactions (Formation of a Precipitate) l One cation of one reactant combine with the anions of another reactant to form insoluble or slightly soluble compound l Example: Potassium iodide + lead (II) nitrate ____ + ____ 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3(aq)
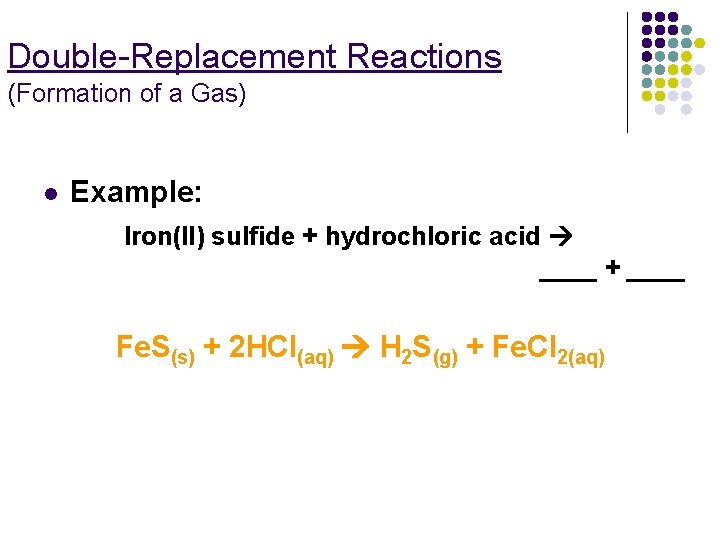
Double-Replacement Reactions (Formation of a Gas) l Example: Iron(II) sulfide + hydrochloric acid ____ + ____ Fe. S(s) + 2 HCl(aq) H 2 S(g) + Fe. Cl 2(aq)
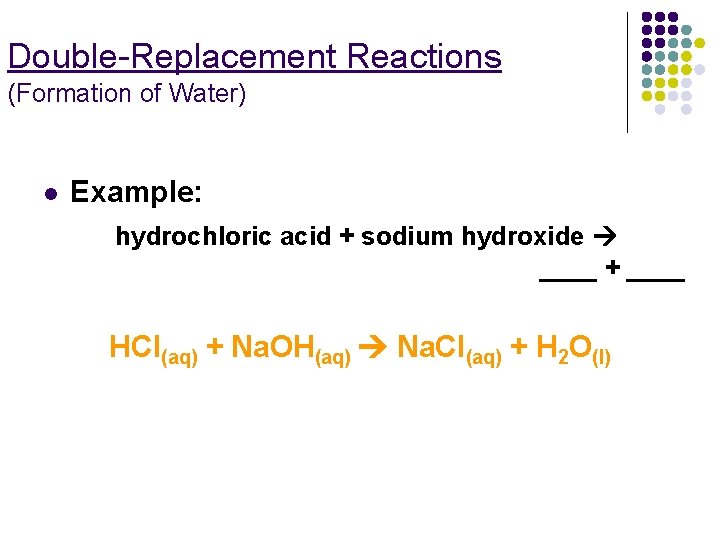
Double-Replacement Reactions (Formation of Water) l Example: hydrochloric acid + sodium hydroxide ____ + ____ HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l)

Combustion Reactions A reaction in which a substance combines with oxygen, releasing a large amount of energy in the form of light and heat Combustion of a hydrocarbon always yield carbon dioxide and water.
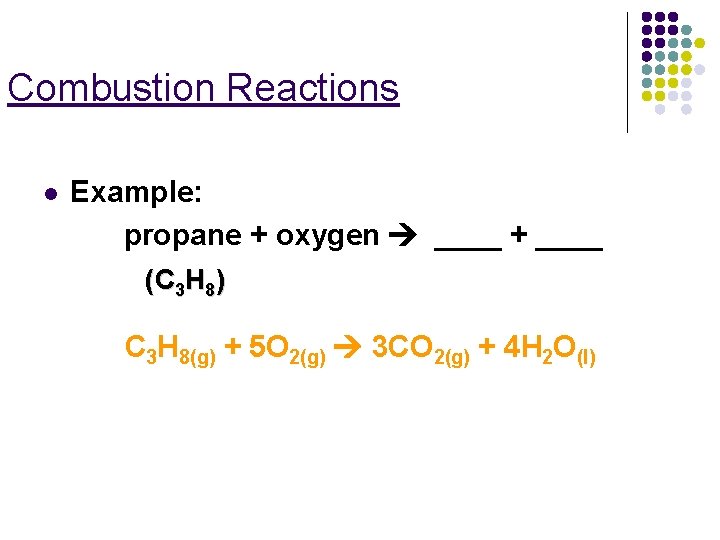
Combustion Reactions l Example: propane + oxygen ____ + ____ (C 3 H 8) C 3 H 8(g) + 5 O 2(g) 3 CO 2(g) + 4 H 2 O(l)

Try this: l Write a balanced chemical equation for the following reaction: Ethyl alcohol (C 2 H 5 OH) burns to produce carbon dioxide and water. C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O

Objectives l l Explain the significance of an activity series. Use an activity series to predict whether a given reaction will occur and what the products will be.

Activity Series l l A list of elements organized according to the ease with which the elements undergo certain chemical reactions Used to help predict whether certain chemical reactions will occur

Activity Series l l Table 8. 3, p. 286 An element can replace any element below it but not above it Based on experiment Will be given to you on test.
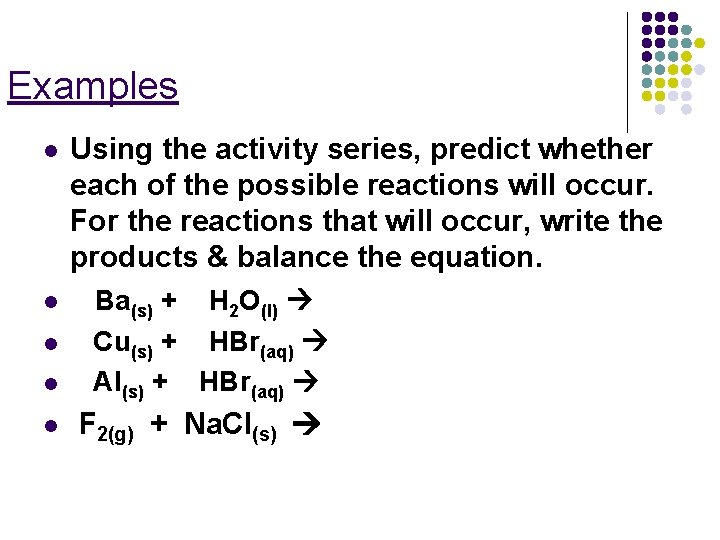
Examples l l l Using the activity series, predict whether each of the possible reactions will occur. For the reactions that will occur, write the products & balance the equation. Ba(s) + H 2 O(l) Cu(s) + HBr(aq) Al(s) + HBr(aq) F 2(g) + Na. Cl(s)
- Slides: 39