Chemical Equations Balancing What is a chemical equation

Chemical Equations & Balancing
What is a chemical equation? • A standard way to describe a chemical reaction. – This means that scientists worldwide use this system. • Show the number and combinations of elements & compounds involved in the reaction.

Chemical Equations … • 2 Parts to a chemical equation: Na. CH 3 COO Na. HCO 3 http: //www. achilles- online. com/catalog/pics/Sodium_ Acetate__Anhydrous. jpg H 2 OCH 3 COOH http: //keetsa. com/blog/wpcontent/uploads/2008/03/ http: //scoutmaster. typepad. com/photos/uncateg orized/2007/03/18/vinegar. jpg http: //www. agoosa. com/images/baking-soda. jpg – Reactants = original substance(s) (substances before any reaction occurs) – Products = created during the reaction (ending substances) CO 2 http: //www. scienceclarified. com/ images/uesc_02_img 0110. jpg
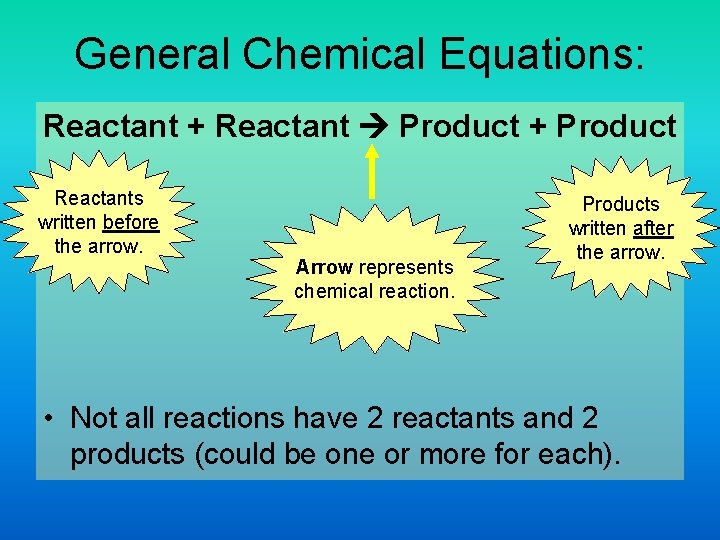
General Chemical Equations: Reactant + Reactant Product + Product Reactants written before the arrow. Arrow represents chemical reaction. Products written after the arrow. • Not all reactions have 2 reactants and 2 products (could be one or more for each).
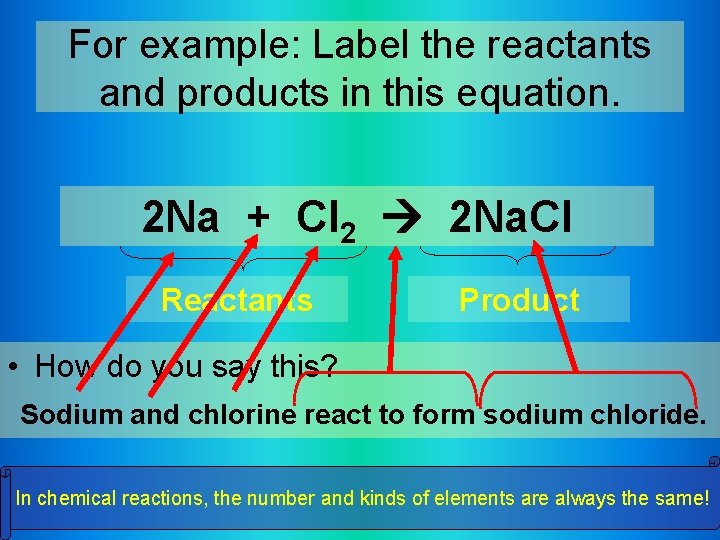
For example: Label the reactants and products in this equation. 2 Na + Cl 2 2 Na. Cl Reactants Product • How do you say this? Sodium and chlorine react to form sodium chloride. In chemical reactions, the number and kinds of elements are always the same!
Chemical Equations also show Conservation of Mass: • There must be the same amount of each element in the reactants as in the products (although the element could be part of different compounds). http: //media. photobucket. com/image/balance%20 scales/nb est 221/scales. jpg

Coefficients: • Used to show conservation of mass in a chemical equation. • Whole number put in front of a reactant or product to show a balanced chemical equation. 2 Na + Cl 2 2 Na. Cl • Multiplies the element(s) found in that specific reactant or product.
Balancing Chemical Equations: • Write each reactant and product. (Remember how ions form chemical bonds. ) • Add coefficients to change the number of atoms within a substance. • Never change the subscripts!
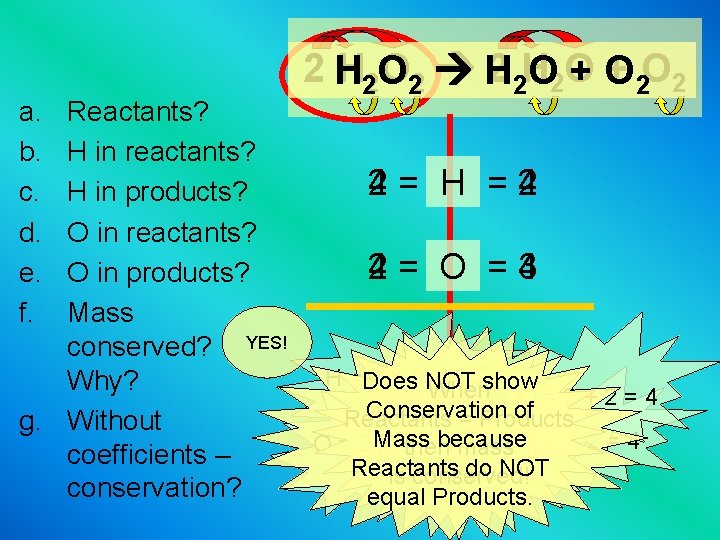
a. b. c. d. e. f. 2 H 22 O 22 H 2 2 H + 2 O 2 O 2 O +O Reactants? H in reactants? 4= H =2 2 4 H in products? O in reactants? 4= O =3 2 4 O in products? Mass conserved? YES! H =Does 2 X 2 NOT = 4 show Why? When O=2+2=4 Conservation of Reactants = Products g. Without Mass because H=2 X 2=4 O = 2 X 2 = 4 mass then coefficients – Reactants do NOT is conserved! conservation? equal Products.
- Slides: 9