Chemical Equations and Reactions Types of Chemical Reactions








- Slides: 11

Chemical Equations and Reactions Types of Chemical Reactions

Types of Chemical Reactions Ø 5 basic types l not all reactions fall in these categories Ø you should be able to: l l categorize a reaction by its reactant(s) predict the product(s)

1. Synthesis Ø also called composition, addition • +

1. Synthesis Ø also called composition, addition Ø combining more than one reactant Ø to make only one product A + X AX A forms a cation (metal) and forms a anion (Non-metal) X

2. Decomposition Ø opposite of synthesis • +

2. Decomposition Ø opposite of synthesis Ø usually require energy Ø breaking apart one reactant Ø to make more than one product AX A + X A is a cation and X is a anion

3. Single Replacement Ø an element replaces a similar element in a compound Ø 1 element & 1 compound as reactants Ø 1 element & 1 compound as products Cation (metal) replaced: A + BX B + AX OR anion (nonmetal) replaced: Y + AX X + AY

3. Single Replacement + +

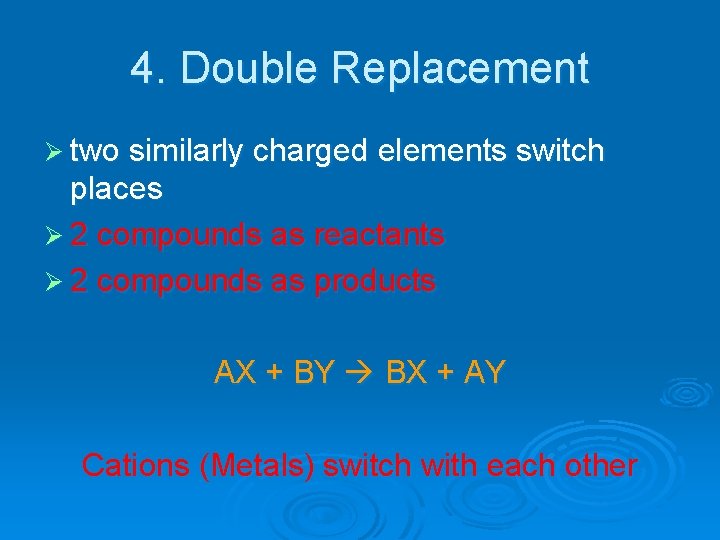
4. Double Replacement

4. Double Replacement Ø two similarly charged elements switch places Ø 2 compounds as reactants Ø 2 compounds as products AX + BY BX + AY Cations (Metals) switch with each other

5. Combustion Ø ALWAYS combines with oxygen, O 2 Ø releases energy in form of heat/light Ø Reactants: Compound (containing H and/or C) + O 2 Ø Products: H 2 O (& usually CO 2) Reactants Products CH 4 + O 2 CO 2 + H 2 O
 Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Are kc and kp equal
Are kc and kp equal Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chemical equations and reactions chapter 8
Chemical equations and reactions chapter 8 Hcl and sodium hydrogen carbonate
Hcl and sodium hydrogen carbonate Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Toxic reactions chemical equations answer key
Toxic reactions chemical equations answer key Chapter 19 chemical reactions simple word equations
Chapter 19 chemical reactions simple word equations Toxic reactions chemical equations
Toxic reactions chemical equations