Chemical Equations A Quick Review Chemical symbol A

Chemical Equations

A Quick Review… • Chemical symbol: A shorthand notation for an element’s name. • Chemical formula: A shorthand notation for a compound or a molecule.

A Quick Review… • Chemical reaction: The process by which one or more substances undergo change to produce one or more different substances. • Writing out a chemical reaction can take a long time and isn’t universal, so there exists a simpler way to represent a chemical reaction called a…
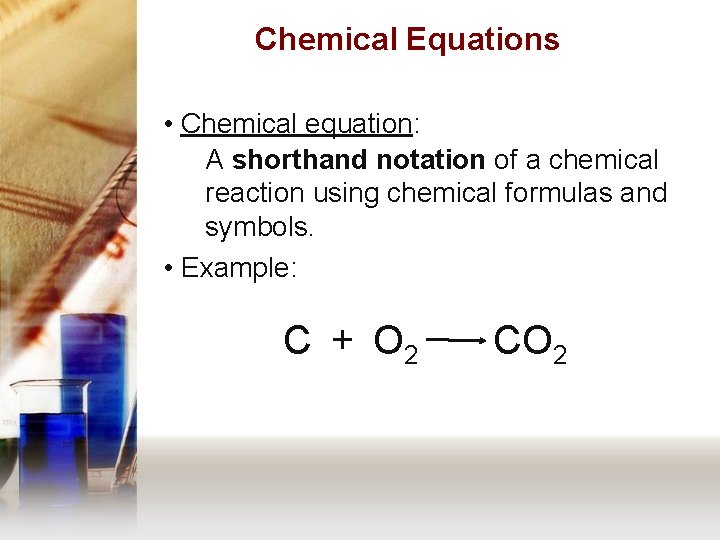
Chemical Equations • Chemical equation: A shorthand notation of a chemical reaction using chemical formulas and symbols. • Example: C + O 2 CO 2
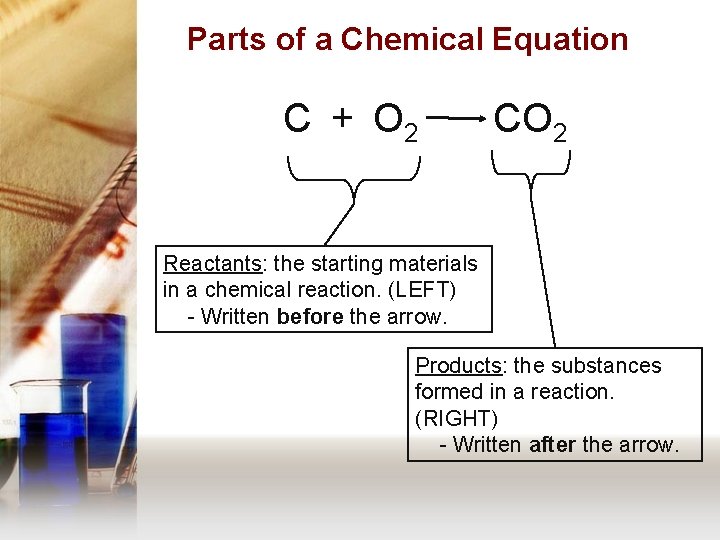
Parts of a Chemical Equation C + O 2 CO 2 Reactants: the starting materials in a chemical reaction. (LEFT) - Written before the arrow. Products: the substances formed in a reaction. (RIGHT) - Written after the arrow.
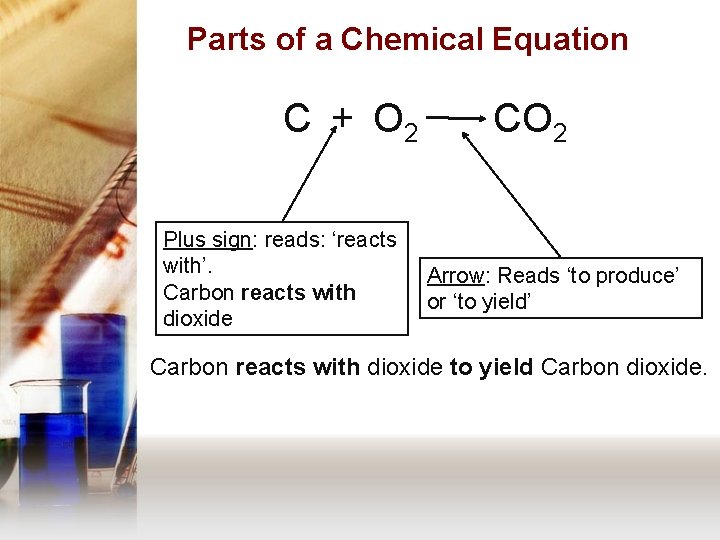
Parts of a Chemical Equation C + O 2 Plus sign: reads: ‘reacts with’. Carbon reacts with dioxide CO 2 Arrow: Reads ‘to produce’ or ‘to yield’ Carbon reacts with dioxide to yield Carbon dioxide.
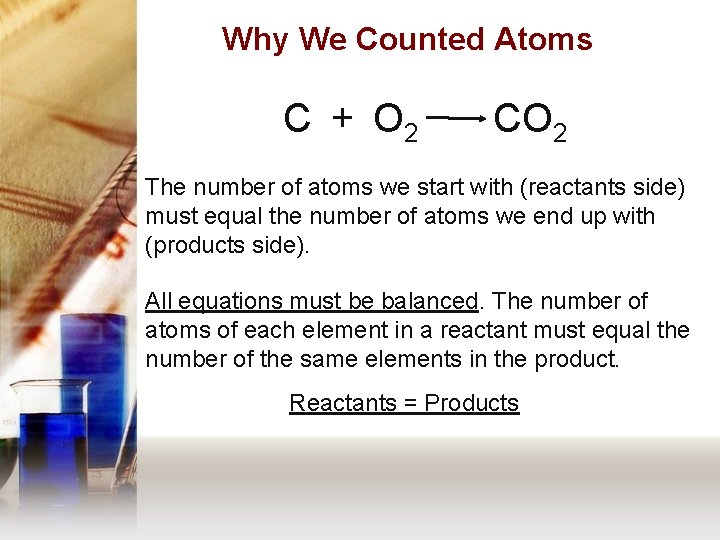
Why We Counted Atoms C + O 2 CO 2 The number of atoms we start with (reactants side) must equal the number of atoms we end up with (products side). All equations must be balanced. The number of atoms of each element in a reactant must equal the number of the same elements in the product. Reactants = Products

Why We Counted Atoms C + O 2 C O 2 When counting atoms for each side, think of the yield sign as an equal sign. C + O 2 = C O 2 • The number of Carbon in the reactants must equal the number of Carbon in the product. • The number of Oxygen in the reactants must equal the number of Oxygen in the product.
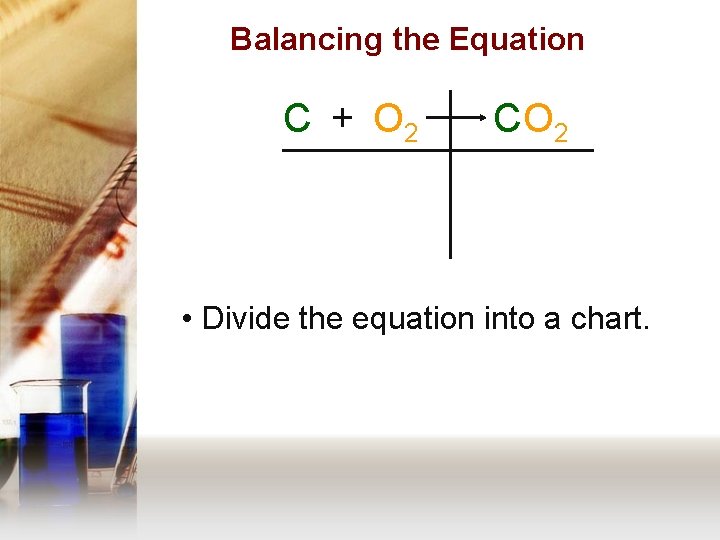
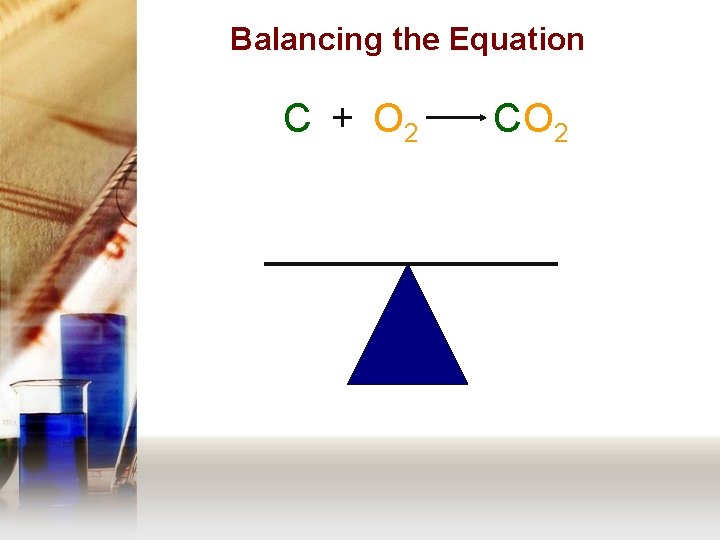
Balancing the Equation C + O 2 C O 2 • Divide the equation into a chart.
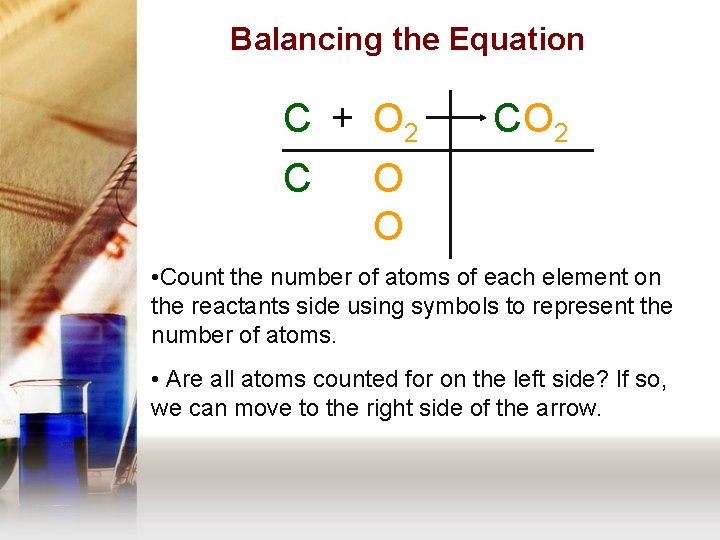
Balancing the Equation C + O 2 C C O 2 O O • Count the number of atoms of each element on the reactants side using symbols to represent the number of atoms. • Are all atoms counted for on the left side? If so, we can move to the right side of the arrow.
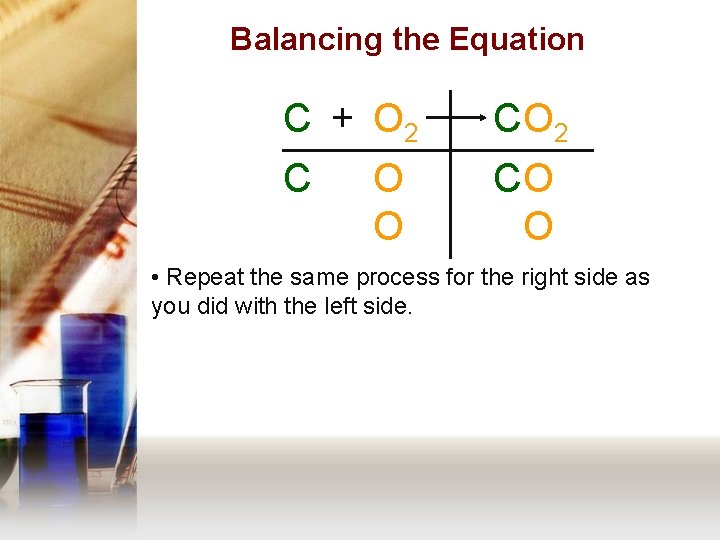
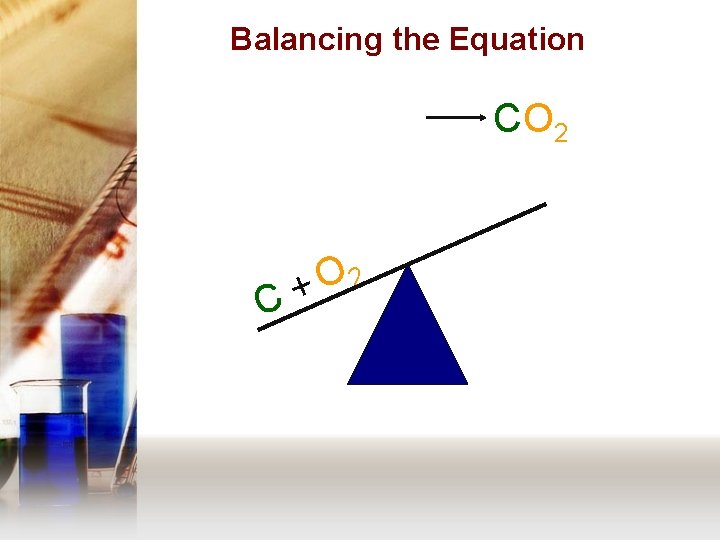
Balancing the Equation C + O 2 C CO O • Repeat the same process for the right side as you did with the left side.
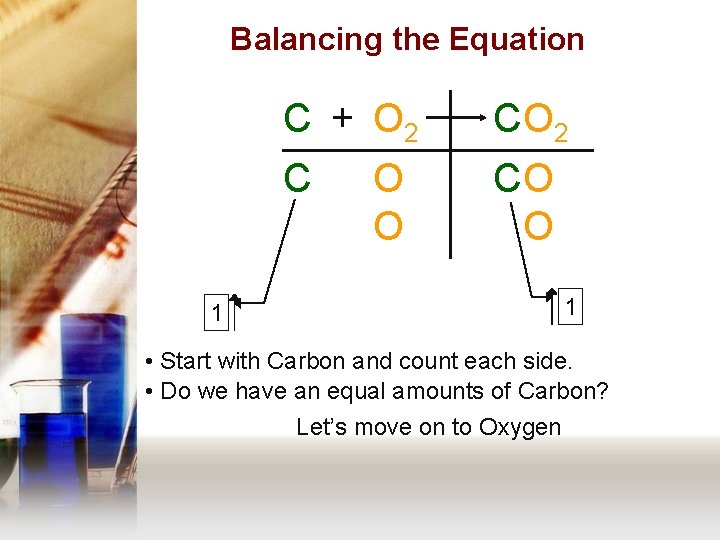
Balancing the Equation 1 C + O 2 C CO O 1 • Start with Carbon and count each side. • Do we have an equal amounts of Carbon? Let’s move on to Oxygen
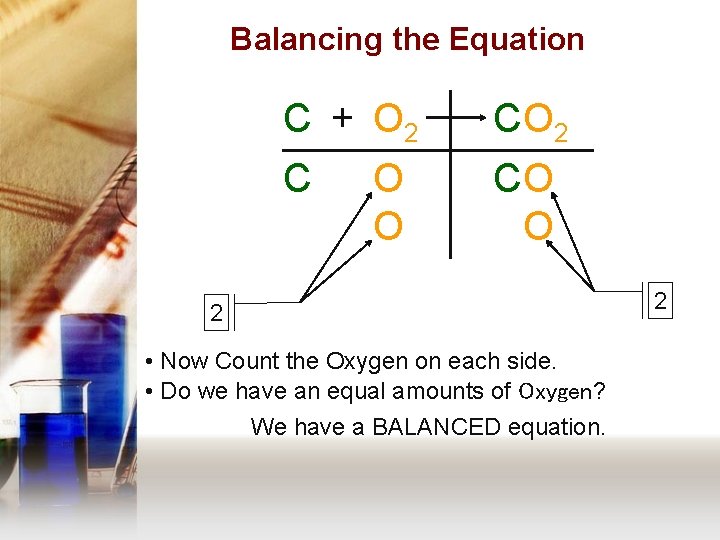
Balancing the Equation C + O 2 C CO O 2 • Now Count the Oxygen on each side. • Do we have an equal amounts of Oxygen? We have a BALANCED equation. 2
Balancing the Equation C + O 2 C O 2
Balancing the Equation C O 2 C 2 O +
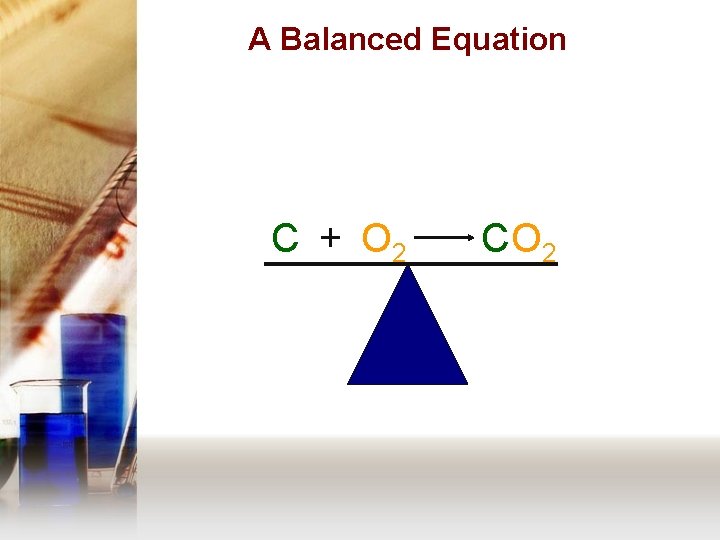
A Balanced Equation C + O 2 C O 2
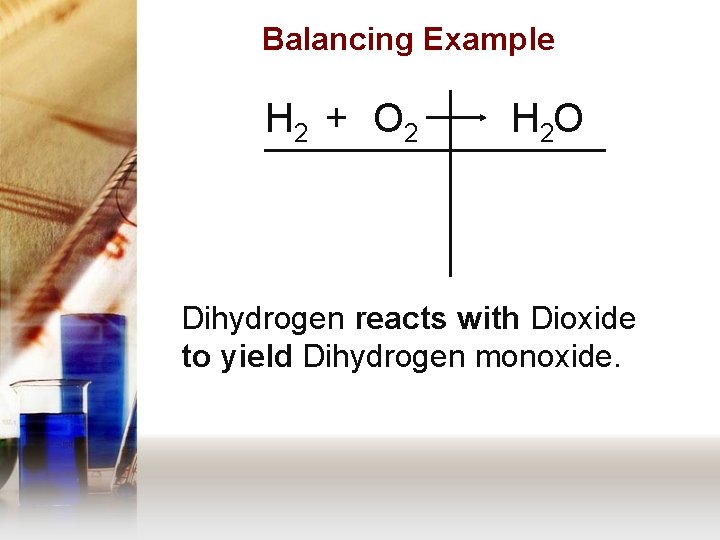
Balancing Example H 2 + O 2 H 2 O Dihydrogen reacts with Dioxide to yield Dihydrogen monoxide.

Balancing Example H 2 + O 2 H H H 2 O O O • Count the number of atoms of each element on the reactants side using symbols to represent the number of atoms.
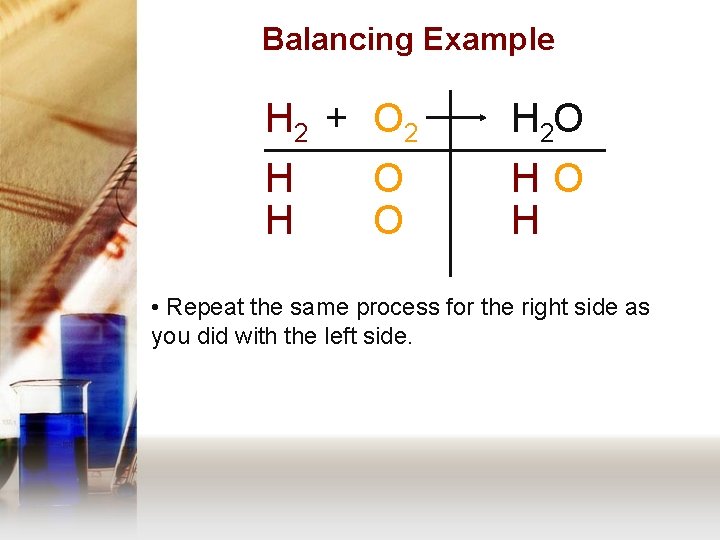
Balancing Example H 2 + O 2 H 2 O H H HO H O O • Repeat the same process for the right side as you did with the left side.
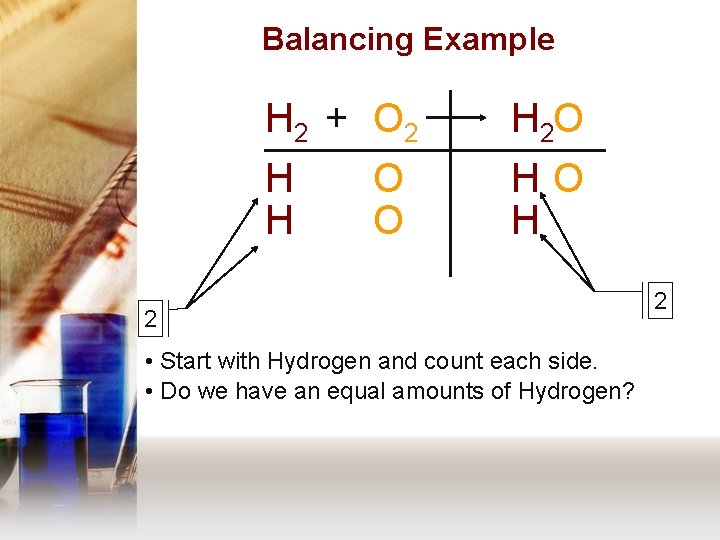
Balancing Example H 2 + O 2 H 2 O H H HO H O O 2 • Start with Hydrogen and count each side. • Do we have an equal amounts of Hydrogen? 2
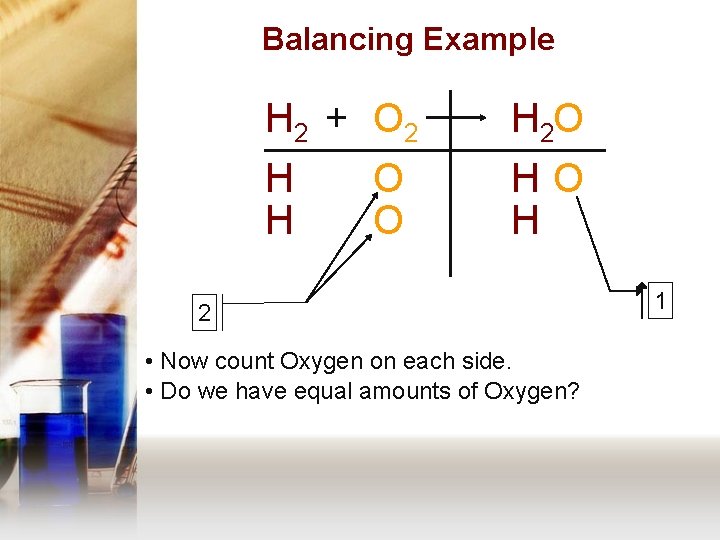
Balancing Example H 2 + O 2 H 2 O H H HO H O O 2 • Now count Oxygen on each side. • Do we have equal amounts of Oxygen? 1
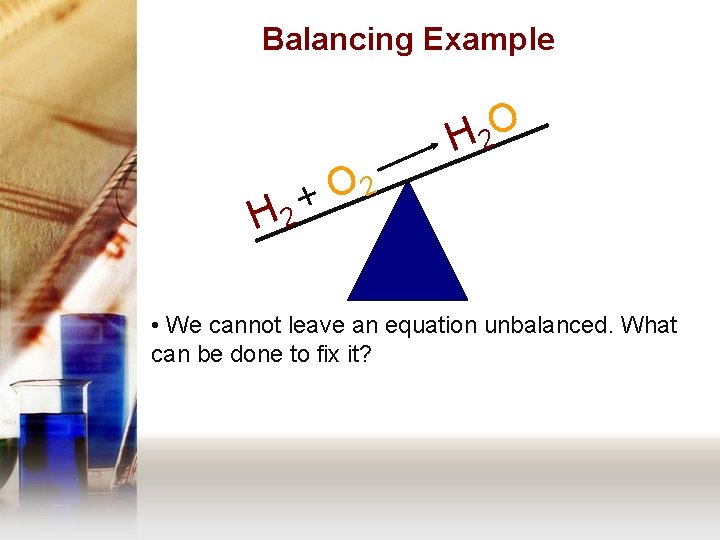
Balancing Example H 2 O H 2 2 O + • We cannot leave an equation unbalanced. What can be done to fix it?
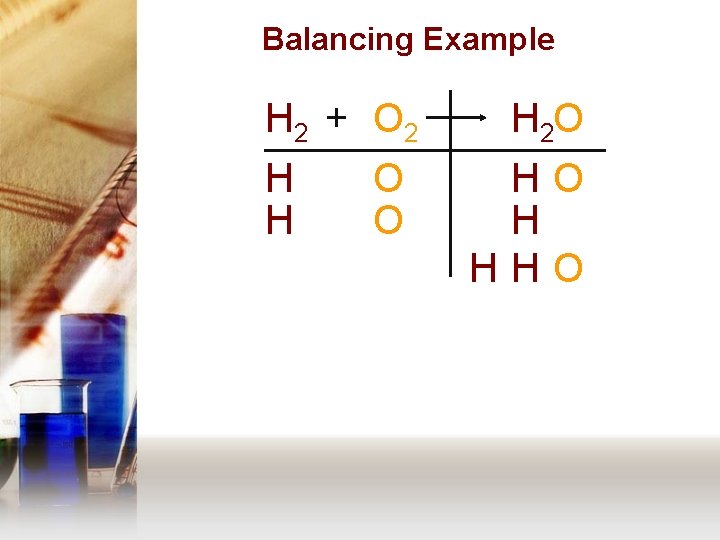
Balancing Example H 2 + O 2 H H O O H 2 O HO H HHO
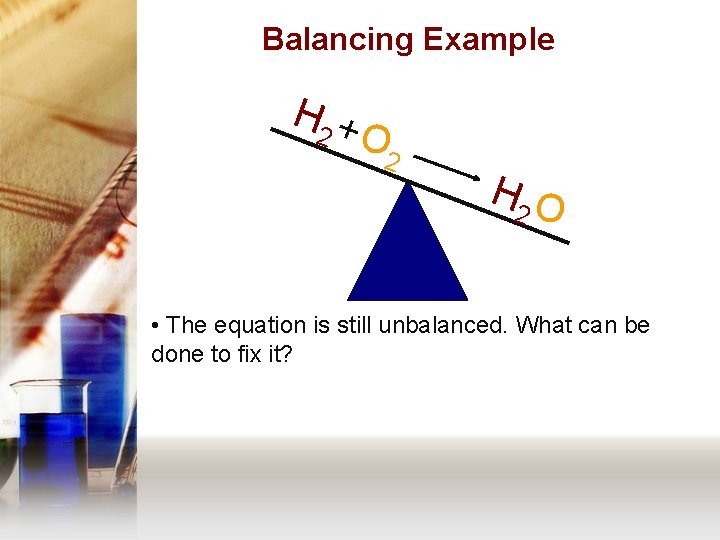
Balancing Example H 2 + O 2 H 2 O • The equation is still unbalanced. What can be done to fix it?
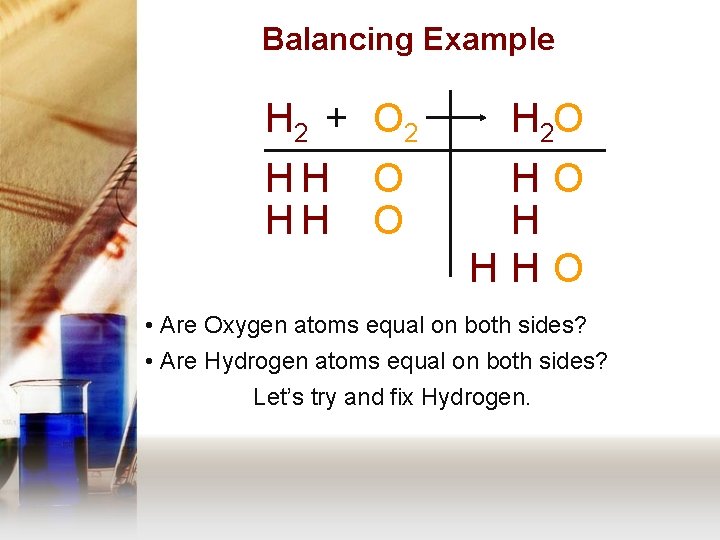
Balancing Example H 2 + O 2 HH HH O O H 2 O HO H HHO • Are Oxygen atoms equal on both sides? • Are Hydrogen atoms equal on both sides? Let’s try and fix Hydrogen.
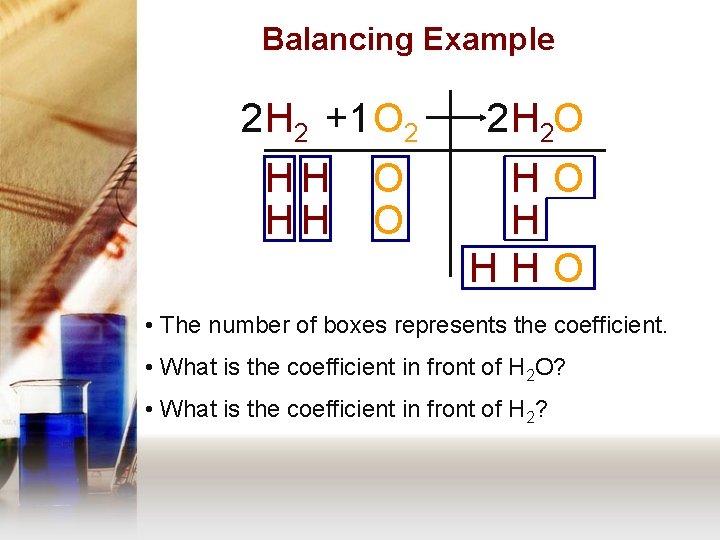
Balancing Example 2 H 2 + 1 O 2 HH HH O O 2 H 2 O HO H HHO • The number of boxes represents the coefficient. • What is the coefficient in front of H 2 O? • What is the coefficient in front of H 2?
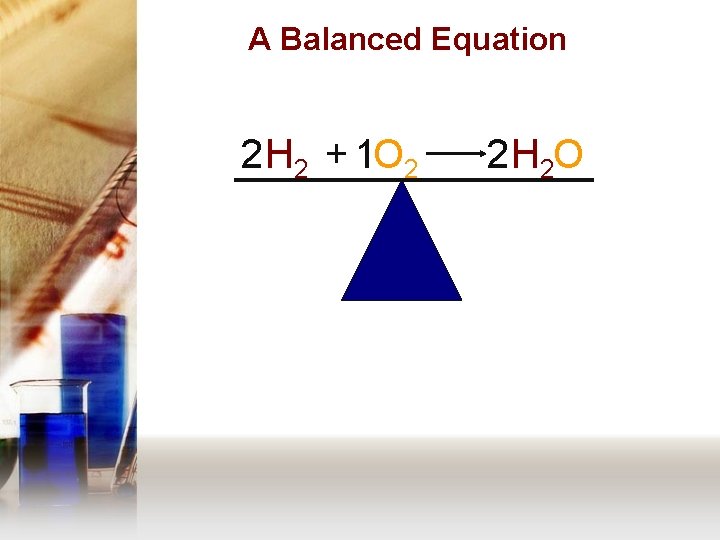
A Balanced Equation 2 H 2 + 1 O 2 2 H 2 O
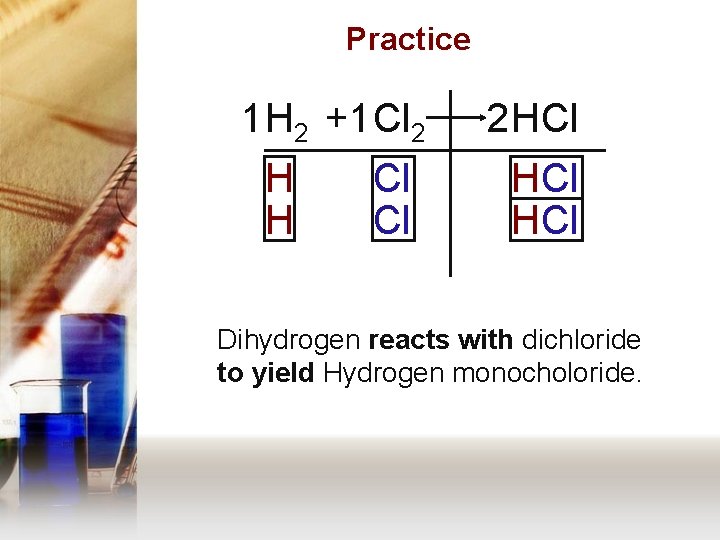
Practice 1 H 2 +1 Cl 2 H H Cl Cl 2 HCl HCl Dihydrogen reacts with dichloride to yield Hydrogen monocholoride.
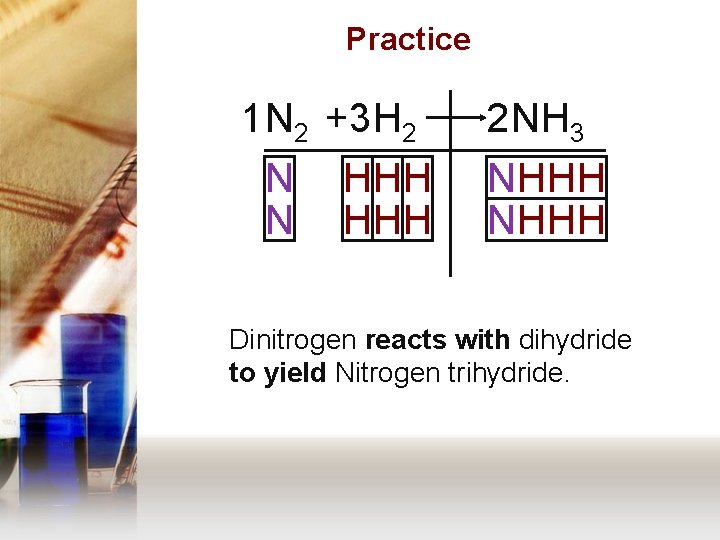
Practice 1 N 2 + 3 H 2 N N HHH 2 N H 3 NHHH Dinitrogen reacts with dihydride to yield Nitrogen trihydride.
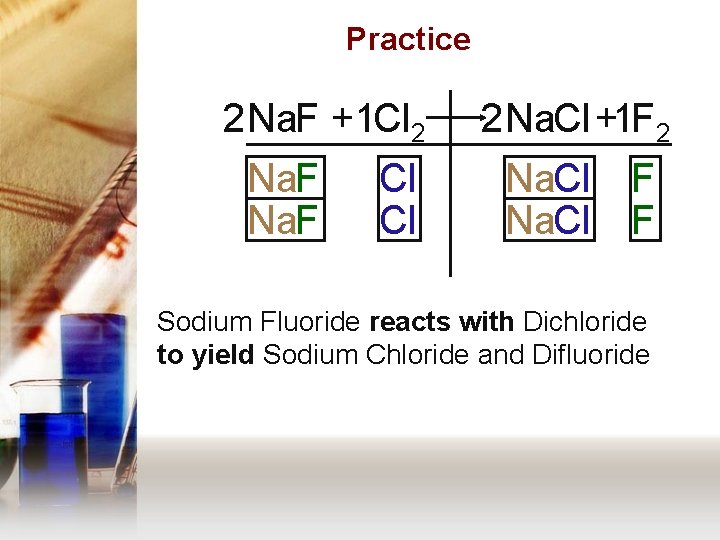
Practice 2 Na. F +1 Cl 2 Na. F Cl Cl 2 Na. Cl +1 F 2 Na. Cl F F Sodium Fluoride reacts with Dichloride to yield Sodium Chloride and Difluoride
- Slides: 30