Chemical Compounds of Life Chapter 4 Organic vs












- Slides: 30

Chemical Compounds of Life Chapter #4

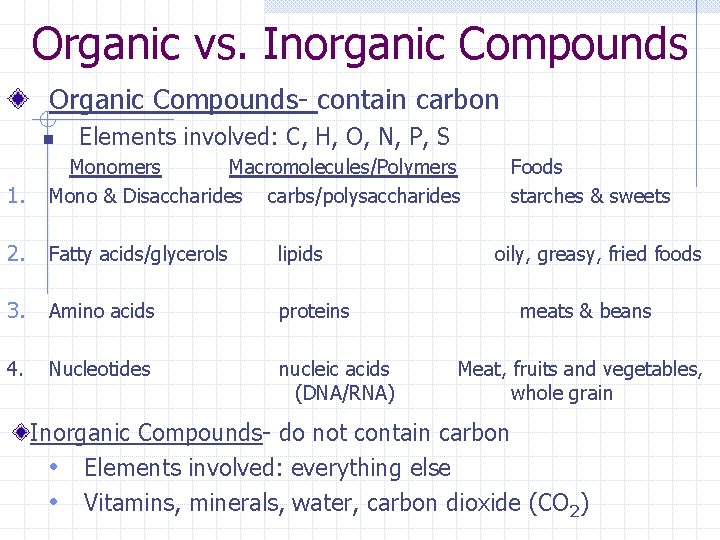
Organic vs. Inorganic Compounds Organic Compounds- contain carbon n Elements involved: C, H, O, N, P, S 1. Monomers Macromolecules/Polymers Mono & Disaccharides carbs/polysaccharides 2. Fatty acids/glycerols lipids 3. Amino acids proteins 4. Nucleotides nucleic acids (DNA/RNA) Foods starches & sweets oily, greasy, fried foods meats & beans Meat, fruits and vegetables, whole grain Inorganic Compounds- do not contain carbon • Elements involved: everything else • Vitamins, minerals, water, carbon dioxide (CO 2)

Water-Important Inorganic Compound All living things need water Many biological processes only occur in water solutions With many unique properties

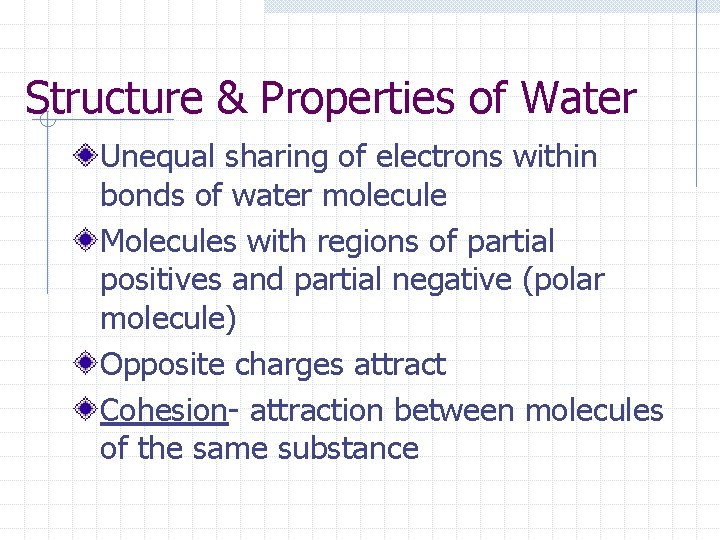
Structure & Properties of Water Unequal sharing of electrons within bonds of water molecule Molecules with regions of partial positives and partial negative (polar molecule) Opposite charges attract Cohesion- attraction between molecules of the same substance

Cohesion Allows water to absorb large amounts of heat without a big change in temp. Protect organisms from overheating! (evaporative cooling)

Adhesion- attraction between the water molecules & molecules of another substance Makes water great solvent

Capillary Action- action of water being drawn up within small glass tube Caused by cohesion and adhesion This is involved with transporting water within plants from the roots to the leaves!!

Capillary Action

Organic Compounds Aside from water, living organisms consist mostly of carbon-based compounds Carbon is unparalleled in its ability to form large, complex, and diverse molecules

Organic Compounds With four valence electrons, carbon can form four covalent bonds with a variety of atoms This ability makes large, complex molecules (chains & rings) possible! Carbon is the “duck tape” of life that is designed to stick together & grab onto other atoms!

Carbs/Sugars- – organic molecules (macro) made of C, H, O (1: 2: 1 ratio) Includes simple sugars & complex sugars

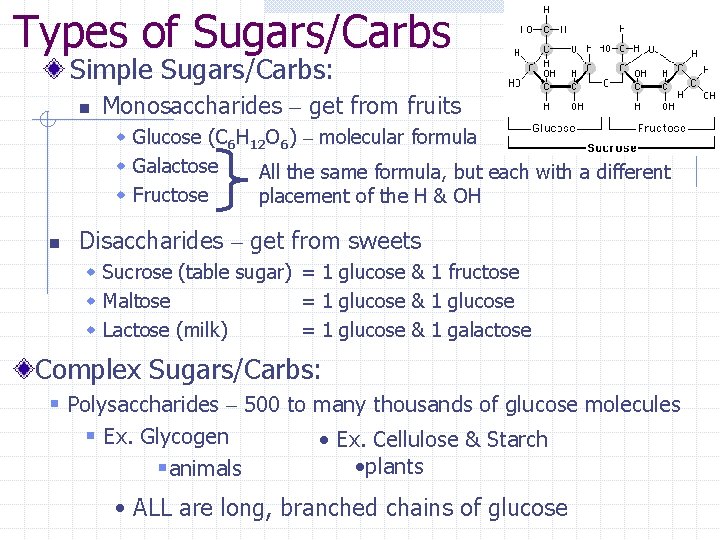
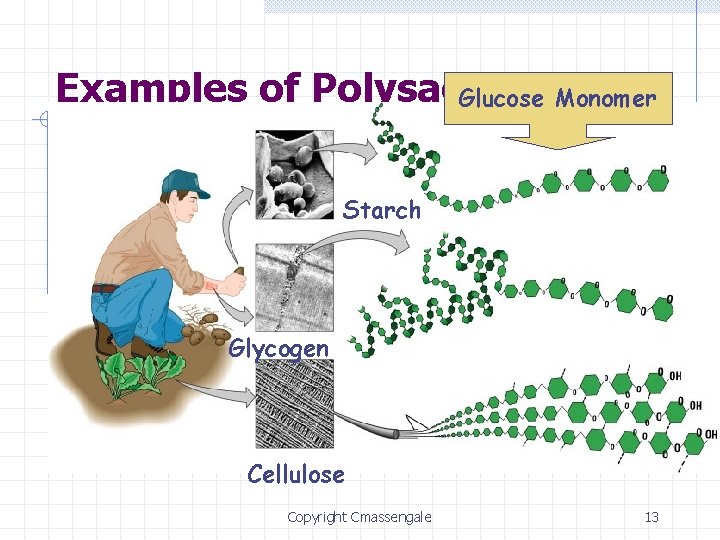
Types of Sugars/Carbs Simple Sugars/Carbs: n Monosaccharides – get from fruits w Glucose (C 6 H 12 O 6) – molecular formula w Galactose w Fructose n All the same formula, but each with a different placement of the H & OH Disaccharides – get from sweets w Sucrose (table sugar) = 1 glucose & 1 fructose w Maltose = 1 glucose & 1 glucose w Lactose (milk) = 1 glucose & 1 galactose Complex Sugars/Carbs: § Polysaccharides – 500 to many thousands of glucose molecules § Ex. Glycogen • Ex. Cellulose & Starch • plants §animals • ALL are long, branched chains of glucose

Examples of Polysaccharides Glucose Monomer Starch Glycogen Cellulose Copyright Cmassengale 13

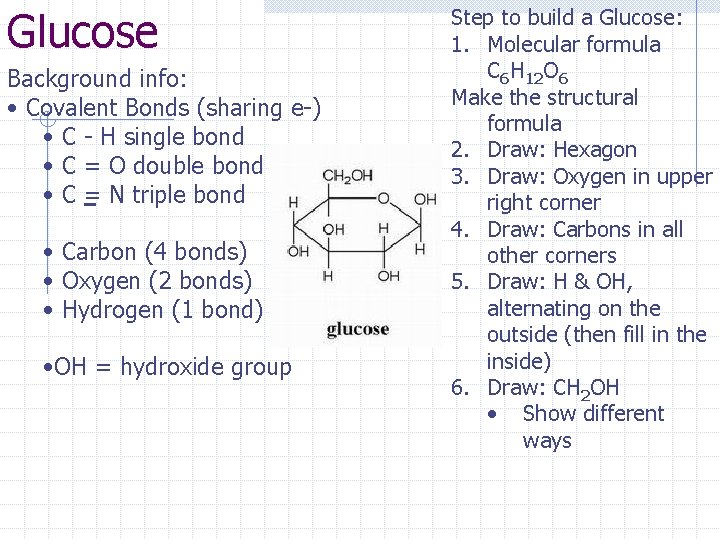
Glucose Background info: • Covalent Bonds (sharing e-) • C - H single bond • C = O double bond • C = N triple bond • Carbon (4 bonds) • Oxygen (2 bonds) • Hydrogen (1 bond) • OH = hydroxide group Step to build a Glucose: 1. Molecular formula C 6 H 12 O 6 Make the structural formula 2. Draw: Hexagon 3. Draw: Oxygen in upper right corner 4. Draw: Carbons in all other corners 5. Draw: H & OH, alternating on the outside (then fill in the inside) 6. Draw: CH 2 OH • Show different ways

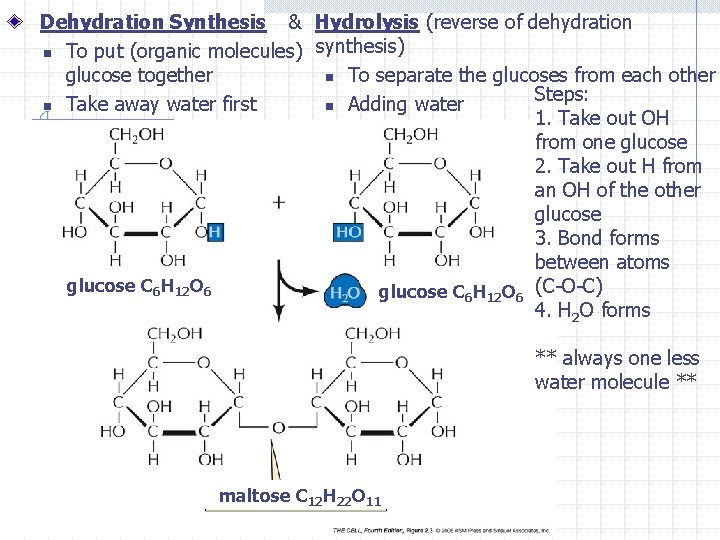
Dehydration Synthesis & Hydrolysis (reverse of dehydration n To put (organic molecules) synthesis) glucose together n To separate the glucoses from each other Steps: n Take away water first n Adding water 1. Take out OH from one glucose 2. Take out H from an OH of the other glucose 3. Bond forms between atoms glucose C 6 H 12 O 6 (C-O-C) 4. H 2 O forms ** always one less water molecule ** maltose C 12 H 22 O 11

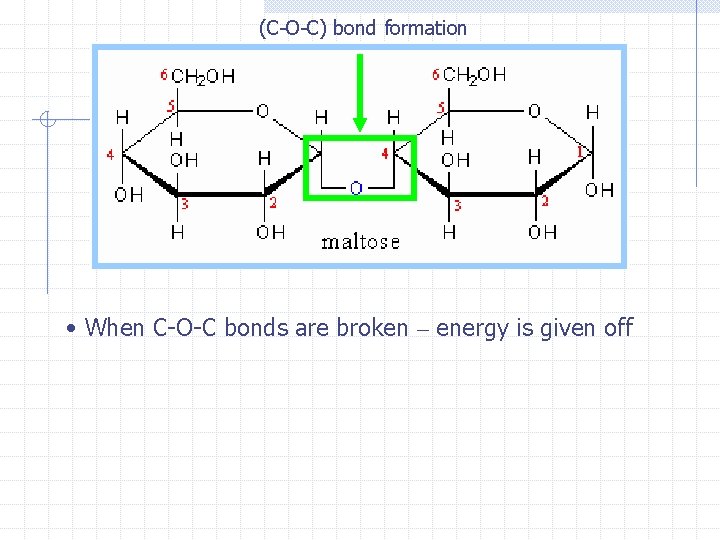
(C-O-C) bond formation • When C-O-C bonds are broken – energy is given off

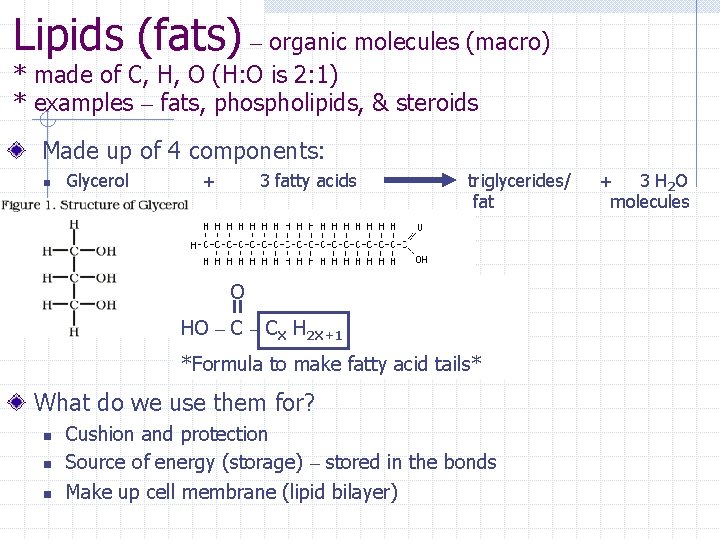
Lipids (fats) – organic molecules (macro) * made of C, H, O (H: O is 2: 1) * examples – fats, phospholipids, & steroids Made up of 4 components: n Glycerol + 3 fatty acids triglycerides/ fat O HO – CX H 2 X+1 *Formula to make fatty acid tails* What do we use them for? n n n Cushion and protection Source of energy (storage) – stored in the bonds Make up cell membrane (lipid bilayer) + 3 H 2 O molecules

Lipids Where do we get them? n n n Oily, greasy foods (fried) Milk, dairy Animal fats (meat) Types of Lipids fats (triglyceride), phospholipids, & steroids Saturated fats- fatty acids with single carbon to carbon bonds (solid at room temp. ) Unsaturated fats- one or more pairs of carbon atoms in fatty acid molecules are joined together by double bonds (liquid at room temp. )

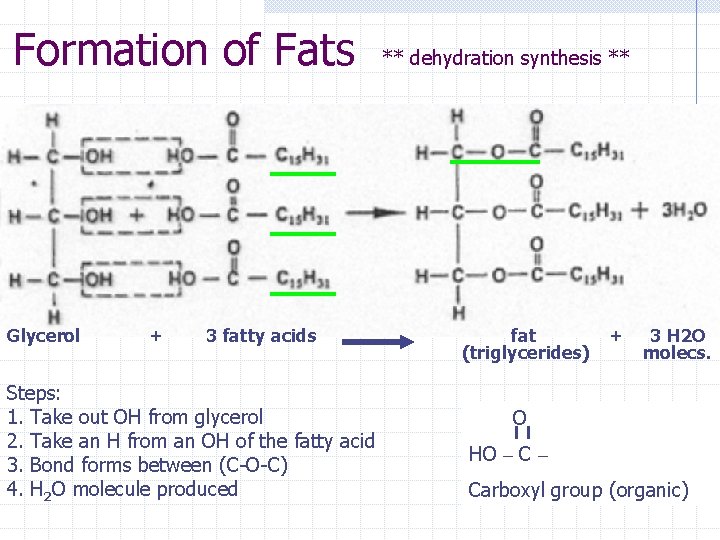
Formation of Fats Glycerol + 3 fatty acids Steps: 1. Take out OH from glycerol 2. Take an H from an OH of the fatty acid 3. Bond forms between (C-O-C) 4. H 2 O molecule produced ** dehydration synthesis ** fat (triglycerides) + 3 H 2 O molecs. O HO – Carboxyl group (organic)

Nucleic Acids Contain C, H, O, N, P DNA (deoxyribonucleic acid) & RNA (ribonucleic acid) DNA & RNA are both composed of a long chain of repeating units (nucleotides) Nucleotide- consist of 5 carbon sugar, nitrogenous base, and a phosphate group

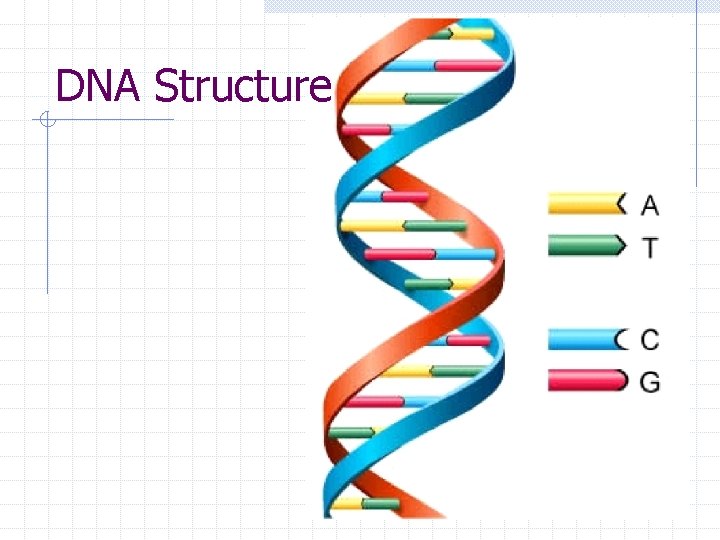
Nucleic Acids DNA nitrogenous bases = adenine, thymine, guanine, and cytosine DNA nucleotides with deoxyribose sugar DNA consists of two strands with double helix shape

DNA Structure

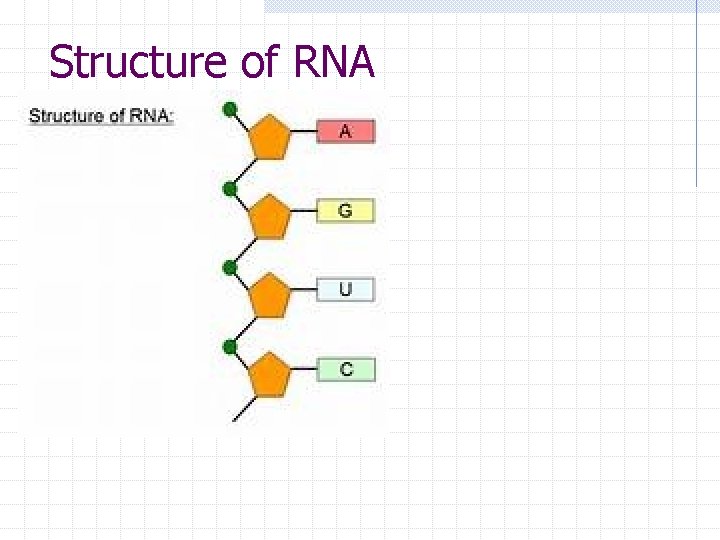
Structure of RNA Single stranded Polymer of nucleotides too! RNA nitrogenous bases: adenine, uracil, guanine, and cytosine RNA nucleotides contain ribose sugar Involved in protein synthesis

Structure of RNA

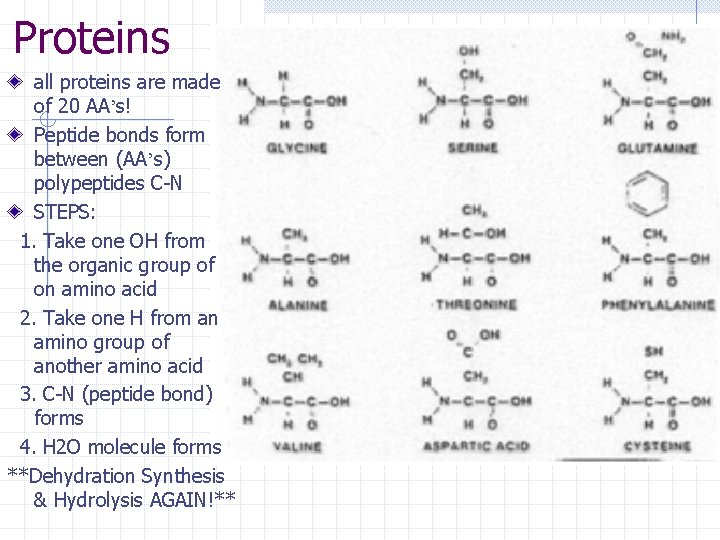
Proteins – organic molecules (macro) * chains of amino acids *made of C, H, O, N, S * examples - enzymes, hormones, antibodies Where do we get them from? n Meats, nuts, legumes (black beans & lentils), fish, beans, dairy, eggs What do we use them for? n Growth, repair, maintenance w Replace tissues in body (cellular level) w Build muscle, enzymes, hormones Made up of 3 components: 1. 2. 3. Nitrogen (amino) group (NH 2) Carboxyl (organic) group (COOH) Amino Acids – 20 different types n Sequence of AA’s makes one protein different from another

Proteins Amino acids- structural units of proteins Peptide bond- bond that forms between amino acids as they are bonding together to form a polypeptide Polypeptide- long chain of amino acids Proteins are made of one or more polypeptides bonded together!

Proteins all proteins are made of 20 AA’s! Peptide bonds form between (AA’s) polypeptides C-N STEPS: 1. Take one OH from the organic group of on amino acid 2. Take one H from an amino group of another amino acid 3. C-N (peptide bond) forms 4. H 2 O molecule forms **Dehydration Synthesis & Hydrolysis AGAIN!**

Enzymes Enzyme- protein substances that are necessary for most chemical reactions to occur within living cells Not changed by reactions Catalyst- substances that bring about a reaction without being changed themselves (enzymes)

Enzyme Function Enzymes have active sites on their surface Substrate molecules fit the shape of the active site When enzyme & substrate come into contact they temporarily bond which could cause substrate to break apart (or may cause two molecules to join) (see p. 69 -70)

What Effects Enzyme Function? Temperature (can cause denaturation) p. H (can cause denaturation) Amount of substrate present Amount of enzyme present Presence of coenzymes to allow enzyme to perform its function