Chemical Compounds in Cells CHEMISTRY OF LIFE Elements








- Slides: 18

Chemical Compounds in Cells

CHEMISTRY OF LIFE • Elements: simplest form of a substance - cannot be broken down any further without changing what it is • Atom: the actual basic unit composed of protons, neutrons, and electrons

THE ATOM • Just like cells are the basic unit of life, the ATOM is the basic unit of matter. • They are very small. If placed side by side one million would stretch a distance. Particle of 1 cm. Charge PROTON • The atom is made up+ of 3 particles. NEUTRON NEUTRAL ELECTRON -

COMPOUNDS • a substance formed by the chemical combination of 2 or more elements in definite proportions – Ex: water, salt, glucose, carbon dioxide • Chemical bonds hold the atoms in a molecule to

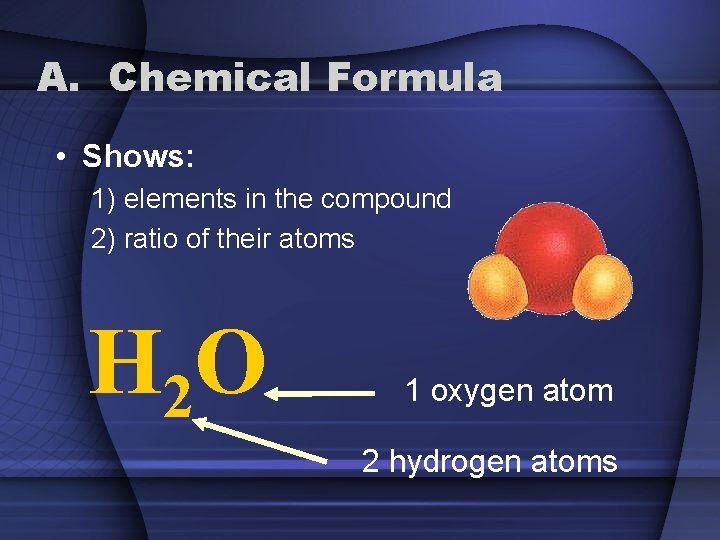
A. Chemical Formula • Shows: 1) elements in the compound 2) ratio of their atoms H 2 O 1 oxygen atom 2 hydrogen atoms

• The cell is a COMPLEX CHEMICAL FACTORY containing some of the same elements found in the nonliving environment. • carbon (C), hydrogen (H), oxygen (O), and nitrogen (N) are present in the greatest percentages

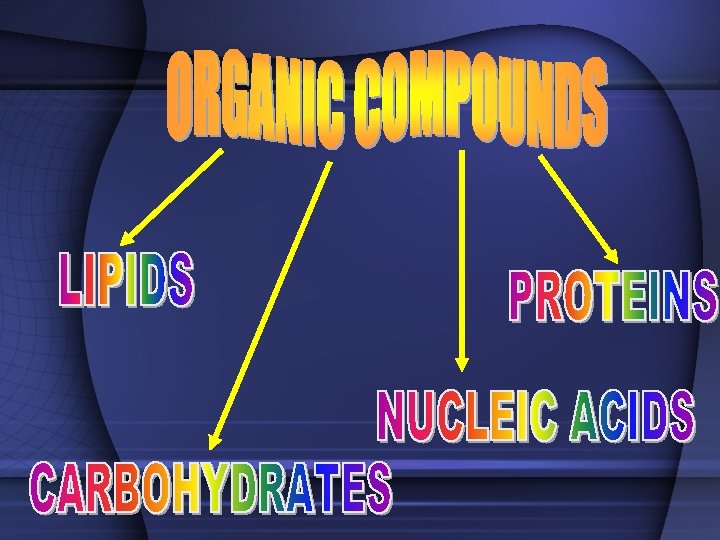
TWO TYPES OF COMPOUNDS • Organic - Contain C, H, and O in some ratio – hydrocarbons (usually referred to as chemicals of life) – Carbohydrates, Proteins, Lipids, Nucleic Acids • Inorganic - usually "support" life – (not hydrocarbons) – Water (H 2 O), Carbon Dioxide (CO 2), Salt

And now for the Biochemistry portion of things….


CARBOHYDRATES • Living things use carbohydrates as a key source of ENERGY! • Plants use carbohydrates for structure (CELLULOSE) – include sugars and complex carbohydrates (starches) – contain the elements carbon, hydrogen, and oxygen

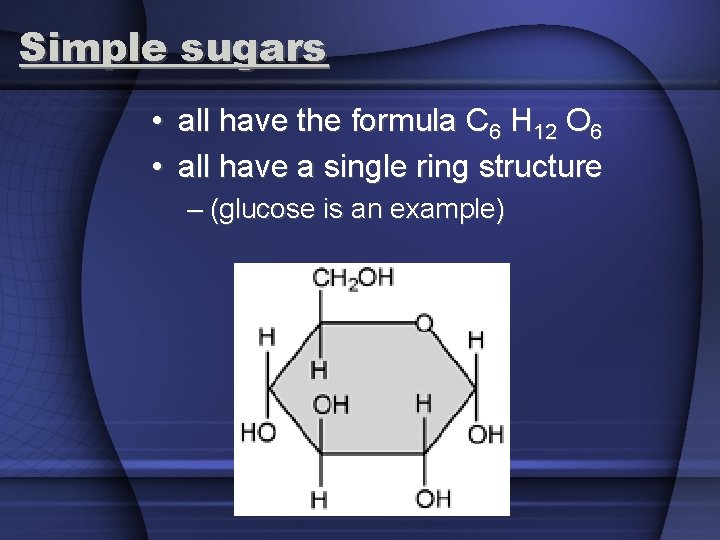
Simple sugars • all have the formula C 6 H 12 O 6 • all have a single ring structure – (glucose is an example)

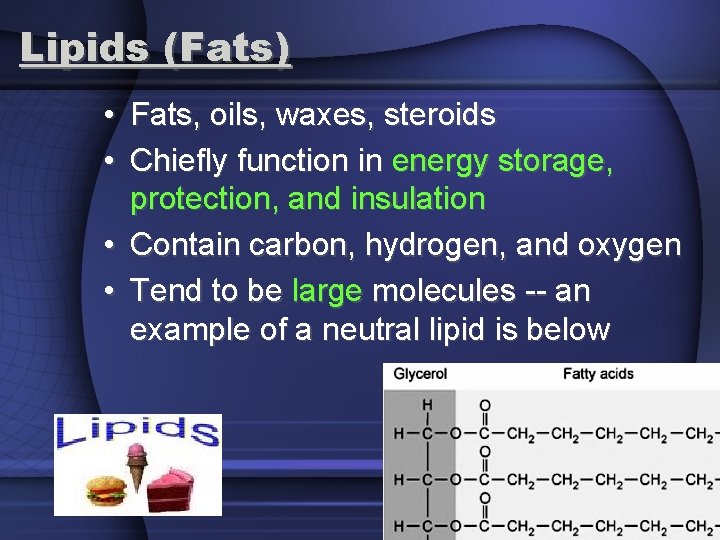
Lipids (Fats) • Fats, oils, waxes, steroids • Chiefly function in energy storage, protection, and insulation • Contain carbon, hydrogen, and oxygen • Tend to be large molecules -- an example of a neutral lipid is below

Lipids • Fats -- found chiefly in animals • Oils and waxes -- found chiefly in plants • Oils are liquid at room temperature, waxes are solids • Lipids along with proteins are key components of cell membranes

PROTEINS • Contain the elements carbon, hydrogen, oxygen, and nitrogen • Composed of amino acids • It is the arrangement of the amino acid that forms the primary structure of proteins. • There are 20 different amino acids that combine in different orders to form different proteins (like letters in the alphabet)

Major Protein Functions • Growth and repair • Energy • Enzymes (help chemical reactions to happen and speed them up)

NUCLEIC ACIDS • in all cells • composed of NUCLEOTIDES • store & transmit heredity/genetic information • Two Types: DNA & RNA

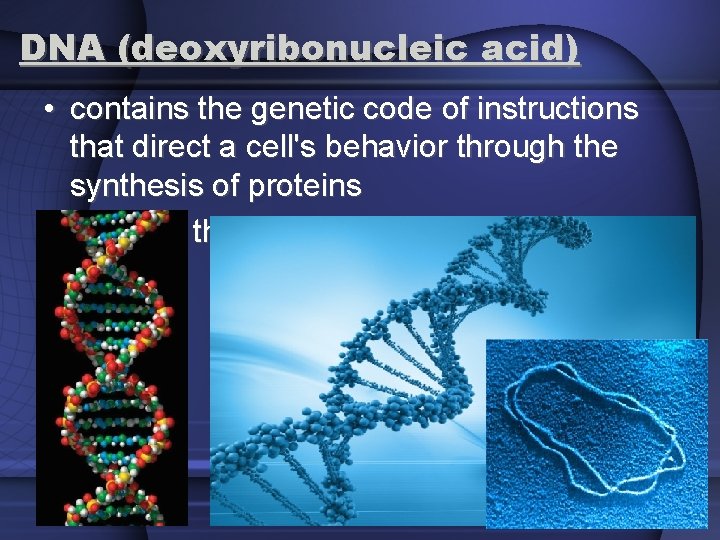
DNA (deoxyribonucleic acid) • contains the genetic code of instructions that direct a cell's behavior through the synthesis of proteins • found in the chromosomes of the nucleus

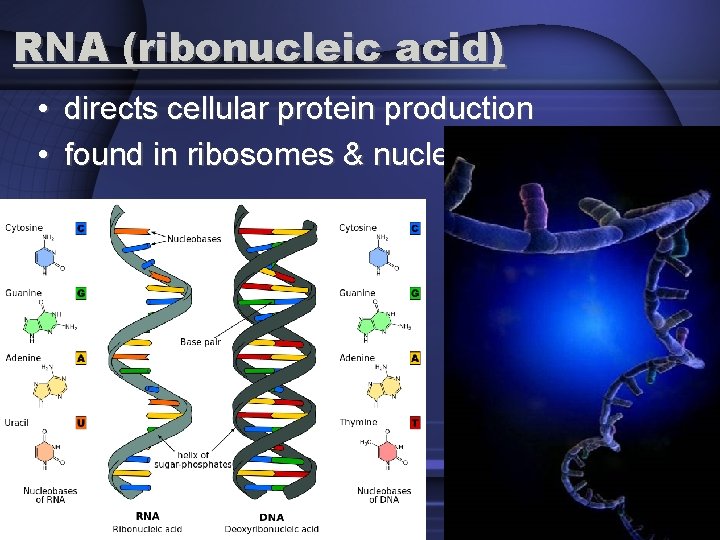
RNA (ribonucleic acid) • directs cellular protein production • found in ribosomes & nucleoli