Chemical compounds are formed by the joining of
Chemical compounds are formed by the joining of two or more atoms. A stable compound occurs when the total energy of the combination has lower energy than the separated atoms. The bound state implies a net attractive force between the atoms. . . a chemical bond. Ionic & Covalent Hydrogen Bond Metallic Bond
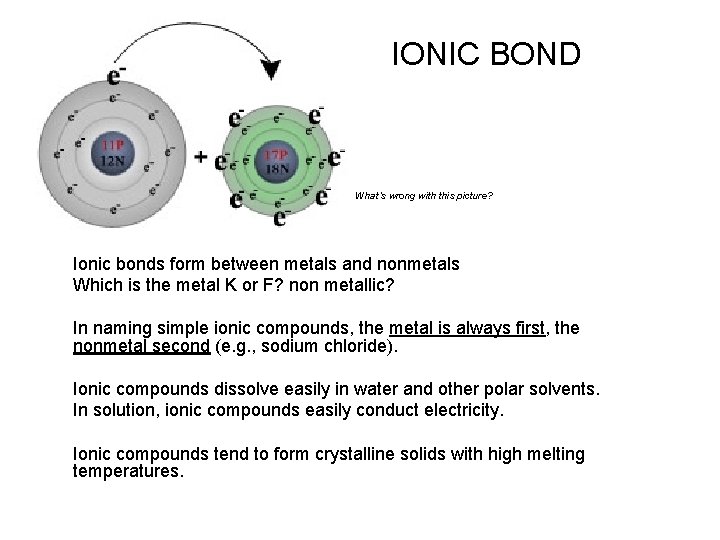
IONIC BOND What’s wrong with this picture? Ionic bonds form between metals and nonmetals Which is the metal K or F? non metallic? In naming simple ionic compounds, the metal is always first, the nonmetal second (e. g. , sodium chloride). Ionic compounds dissolve easily in water and other polar solvents. In solution, ionic compounds easily conduct electricity. Ionic compounds tend to form crystalline solids with high melting temperatures.
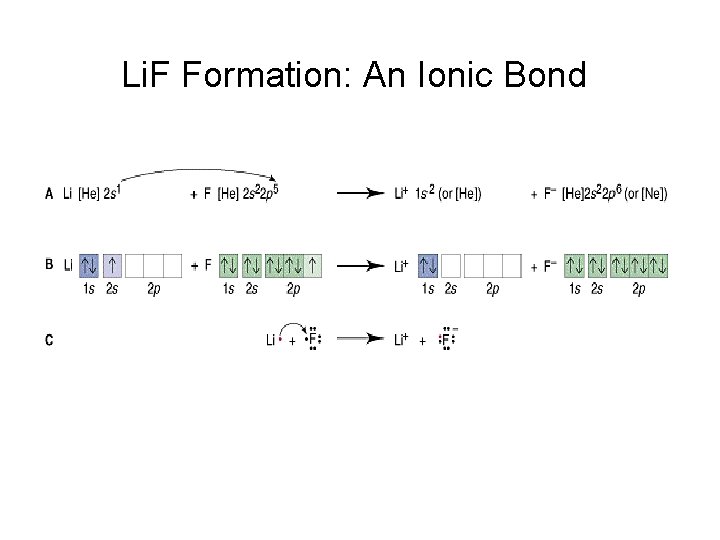
Li. F Formation: An Ionic Bond
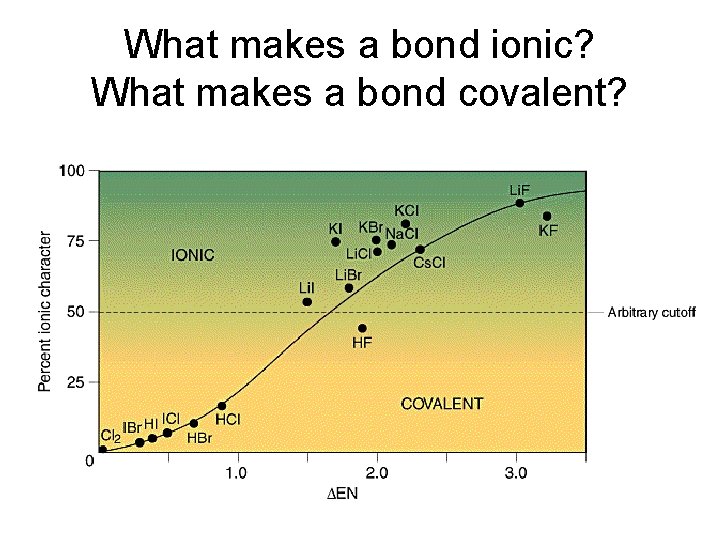
What makes a bond ionic? What makes a bond covalent?
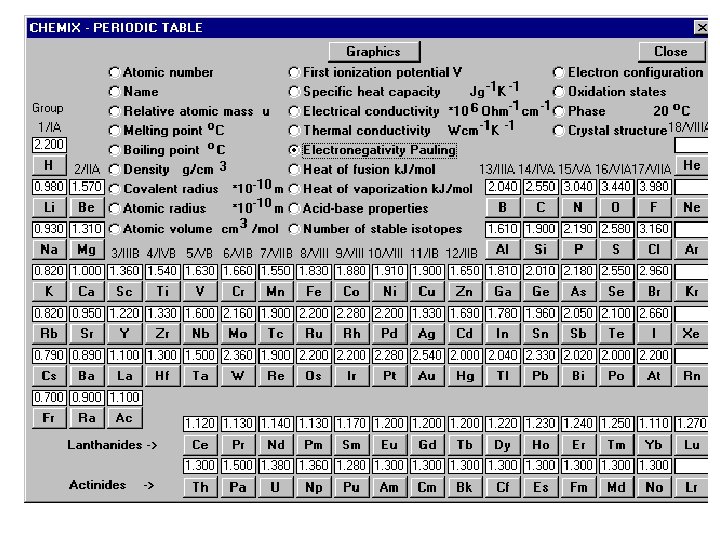
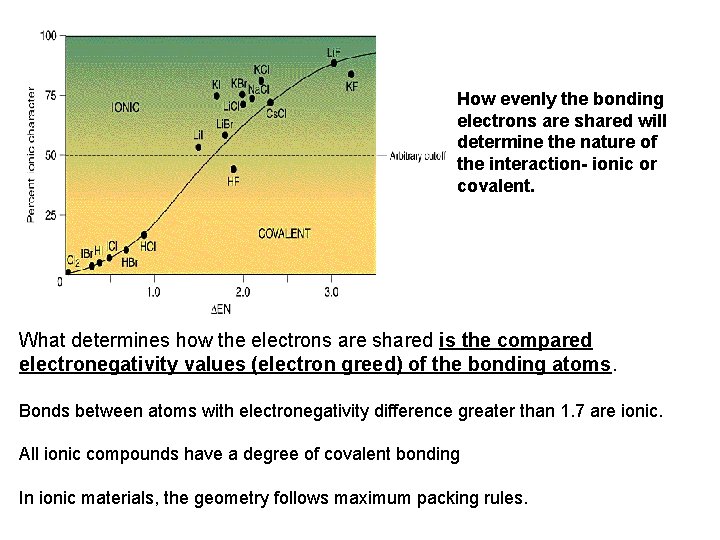
How evenly the bonding electrons are shared will determine the nature of the interaction- ionic or covalent. What determines how the electrons are shared is the compared electronegativity values (electron greed) of the bonding atoms. Bonds between atoms with electronegativity difference greater than 1. 7 are ionic. All ionic compounds have a degree of covalent bonding In ionic materials, the geometry follows maximum packing rules.
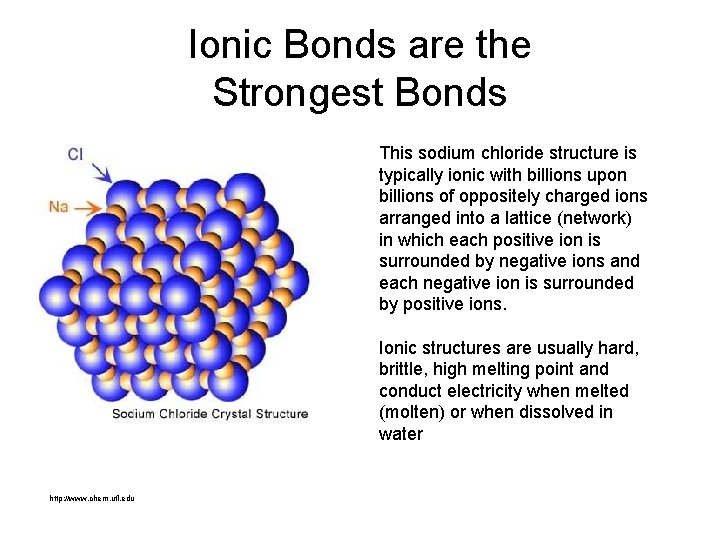
Ionic Bonds are the Strongest Bonds This sodium chloride structure is typically ionic with billions upon billions of oppositely charged ions arranged into a lattice (network) in which each positive ion is surrounded by negative ions and each negative ion is surrounded by positive ions. Ionic structures are usually hard, brittle, high melting point and conduct electricity when melted (molten) or when dissolved in water http: //www. chem. ufl. edu

Could the following bond ionically? Magnesium and Oxygen Mg and Cl Write out the electronic configuration for each atom and show a potential model for ionic bonding.
- Slides: 8