Chemical Chemical Reactions Combination of two or more
Chemical
Chemical Reactions • Combination of two or more elements bonded together to make a new substance. • Example: –Na + Cl = Na. Cl
Real-Life Examples Evidence of CR Examples of CR • Production of gas • Change of color • Change in energy (heat/light) • Odor (gas formed/can’t see) • Precipitate is formed • • Fire/explosions Fireworks Making salt Stomach digesting food – Substance forms on top of a solution. https: //www. youtube. com/watch? v=0 KFAoq. ODZok

CHEMICAL EQUATIONS
CHEMICAL EQUATIONS • A Chemical Equation is a representation of a chemical reaction expressed as a formula. • An Example of a Chemical Equation is when Carbon and Oxygen react to produce Carbon Dioxide. • Carbon + Oxygen = Carbon Dioxide • C + O 2 CO 2
CHEMICAL EQUATIONS • The substances that change in a reaction are called the reactants: • C + O 2 CO 2 • The new substances that are formed as a result of the reaction are called the products: • C + O 2 CO 2 • The arrow represents the energy needed to complete the reaction.
CHEMICAL EQUATIONS • Many equations have subscripts. • Subscripts are numbers that appear after elements in a chemical reactions that represents the number of each atom present. • Example: • H 2 + O 2 H 20 • N 2 + H 2 NH 3
CHEMICAL EQUATIONS • Some equations have a coefficient. • Coefficients are numbers that appear before elements in a chemical equation that change the number of reactants or products. • Example: • H 2 + O 2 H 20 • The correct way to write this equation is: • 2 H 2 + O 2 2 H 2 O • The coefficients change the number of hydrogen and water molecules present.

1. The combination of two or more elements bonded together to make a new substance is called a A. Chemical Reaction B. Coefficient C. Subscript D. Element

2. Which of the following is not considered evidence of a chemical reaction? A. Formation of a precipitate B. Production of a gas C. Change in color D. Cutting a piece of paper in half

3. A chemical equation is A. Numbers that appear after elements in a chemical reaction that represent the number of each atom present. B. A representation of a chemical reaction expressed as a formula. C. Substances that change in a reaction. D. The new substances that are formed as a result of the reaction.
4. In a chemical equation, the coefficients are A. Carbon and Oxygen B. The new substances that are formed as a result of the reaction. C. Numbers that appear before elements in a chemical equation that change the number of reactants or products. D. Substances that change in a chemical reaction.
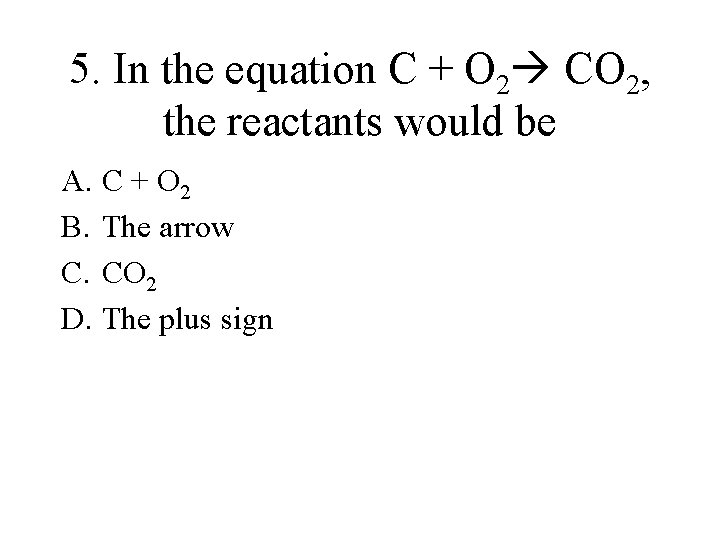
5. In the equation C + O 2 CO 2, the reactants would be A. C + O 2 B. The arrow C. CO 2 D. The plus sign
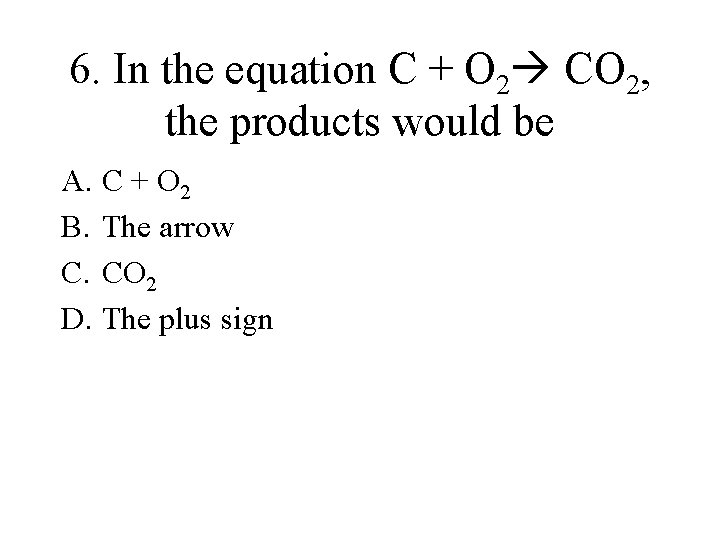
6. In the equation C + O 2 CO 2, the products would be A. C + O 2 B. The arrow C. CO 2 D. The plus sign

COUNTING ATOMS

RULES FOR COUNTING ATOMS 1. SUBSCRIPTS only refer to the atom that they are BEHIND. For example… H 2 S There are TWO atoms of HYDROGEN and only ONE atom of SULFUR.
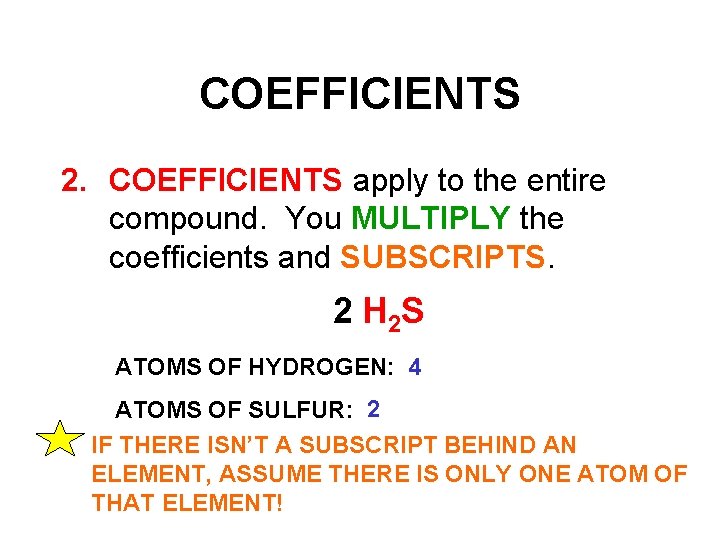
COEFFICIENTS 2. COEFFICIENTS apply to the entire compound. You MULTIPLY the coefficients and SUBSCRIPTS. 2 H 2 S ATOMS OF HYDROGEN: 4 ATOMS OF SULFUR: 2 IF THERE ISN’T A SUBSCRIPT BEHIND AN ELEMENT, ASSUME THERE IS ONLY ONE ATOM OF THAT ELEMENT!
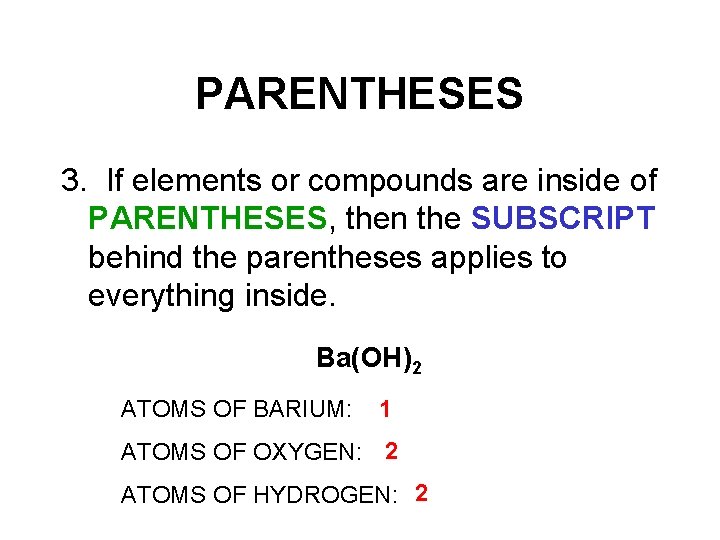
PARENTHESES 3. If elements or compounds are inside of PARENTHESES, then the SUBSCRIPT behind the parentheses applies to everything inside. Ba(OH)2 ATOMS OF BARIUM: 1 ATOMS OF OXYGEN: 2 ATOMS OF HYDROGEN: 2

LET’S PRACTICE! Mg. Cl 2 Atoms of Magnesium: 1 Atoms of Chlorine: 2 Al 2 S 3 Atoms of Aluminum: 2 Atoms of Sulfur: 3
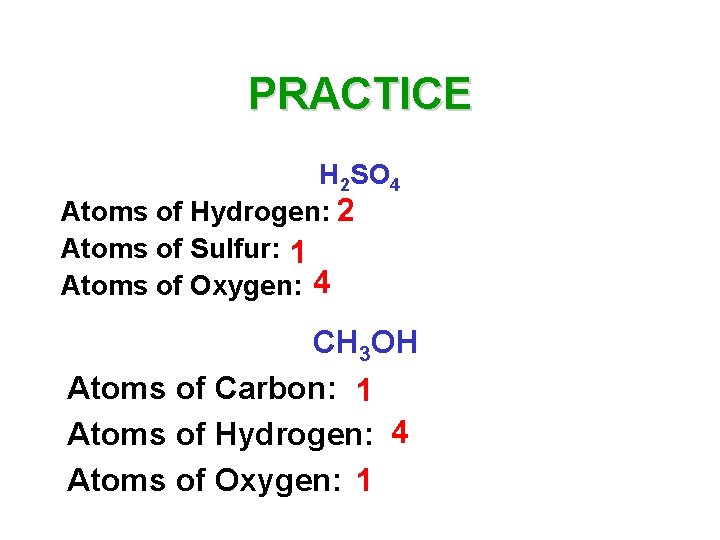
PRACTICE H 2 SO 4 Atoms of Hydrogen: 2 Atoms of Sulfur: 1 Atoms of Oxygen: 4 CH 3 OH Atoms of Carbon: 1 Atoms of Hydrogen: 4 Atoms of Oxygen: 1

THIS COULD BE A LITTLE TRICKY… Ca 3(PO 4)2 Atoms of Calcium: 3 Atoms of Phosphorus: Atoms of Oxygen: 8 2 Al 2(SO 4)3 Atoms of Aluminum: 2 Atoms of Sulfur: 3 Atoms of Oxygen: 12
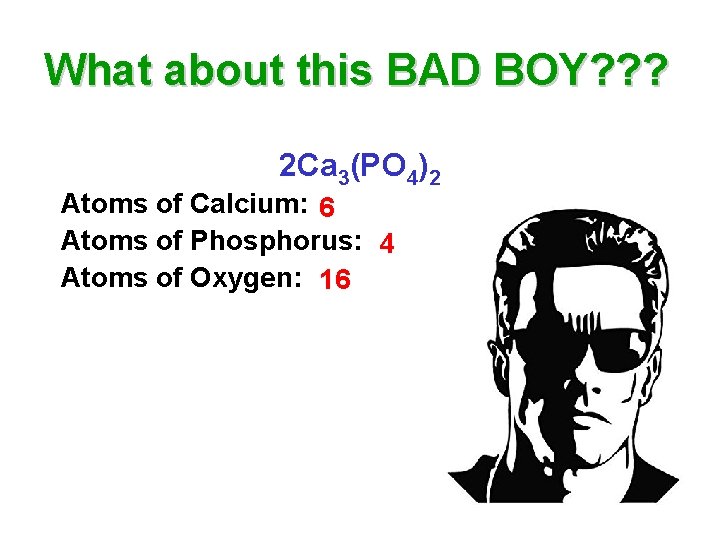
What about this BAD BOY? ? ? 2 Ca 3(PO 4)2 Atoms of Calcium: 6 Atoms of Phosphorus: 4 Atoms of Oxygen: 16
- Slides: 22