Chemical Change Chapter 3 Section 3 1 Objectives

Chemical Change Chapter 3 Section 3. 1

Objectives � Identify important reactions in society � Recognize and identify evidence for chemical changes � Differentiate between endothermic and exothermic reactions � Describe the Law of Conservation of Mass

To Start � What is the difference between a chemical and physical change? ◦ Chemical change- something new is created with its own unique properties ◦ Physical change- nothing new is created (just changing states) �What are three states?

Chemical or Physical? � Which of the following are chemical changes? Physical changes?
Chemical Change � Reactant + reactant product(s) � Products have different properties than reactants ◦ Properties include: state at RT, temperature, melting point, color and density � Includes a flow of energy ◦ IMPORTANT: drives chemical reactions � Can be fast or slow

Examples of Chemical Reactions � Batteries � Combustion Engine � Wine production � Baking bread � Photosynthesis � Cellular respiration (making energy in our bodies)
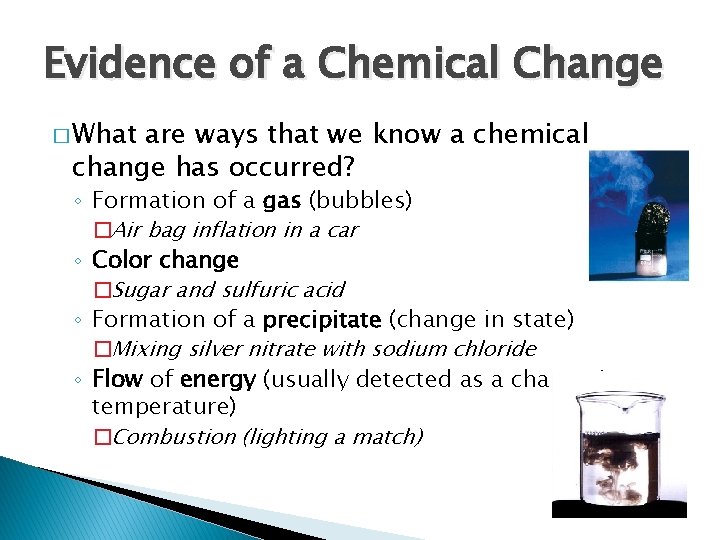
Evidence of a Chemical Change � What are ways that we know a chemical change has occurred? ◦ Formation of a gas (bubbles) �Air bag inflation in a car ◦ Color change �Sugar and sulfuric acid ◦ Formation of a precipitate (change in state) �Mixing silver nitrate with sodium chloride ◦ Flow of energy (usually detected as a change in temperature) �Combustion (lighting a match)

Formation of a Gas: Demo � What will happen when I add baking soda to vinegar in this beaker? � What will happen to the balloon placed over the top? � What gas is produced? � What other examples do we have of a formation of a gas?
Energy Changes � Two types of energy changes: ◦ Exothermic- release of energy (*exit) ◦ Endothermic- absorption of energy (*enter) � What change of temperature would you feel with each of these processes? � Can physical changes be exothermic and endothermic? Why or why not?
Exothermic Reactions � Release energy, usually as: ◦ heat (flame) ◦ light (bioluminescence) ◦ Electricity (battery) � Important ex. : Combustion ◦ What is combustion? � Combustion- oxygen reacts rapidly with another substance, releasing energy (burning) ◦ 2 C 6 H 14(l) + O 2(g) 12 CO 2(g) + 14 H 2 O(g) + energy ◦ (Combustion of hexane)
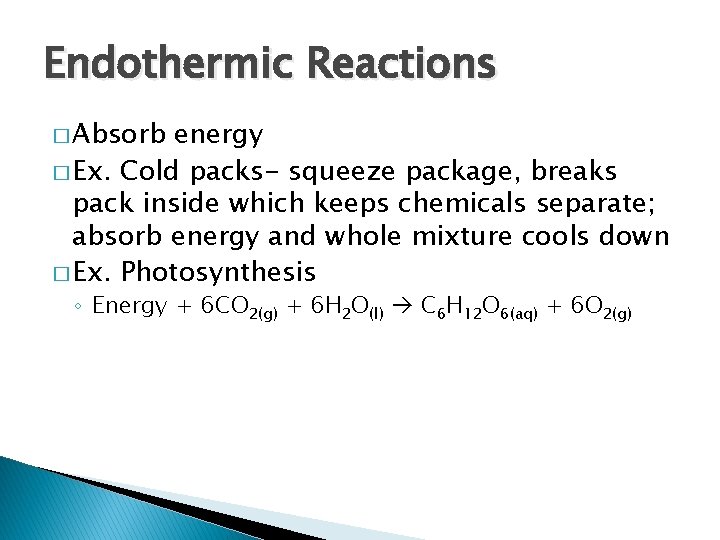
Endothermic Reactions � Absorb energy � Ex. Cold packs- squeeze package, breaks pack inside which keeps chemicals separate; absorb energy and whole mixture cools down � Ex. Photosynthesis ◦ Energy + 6 CO 2(g) + 6 H 2 O(l) C 6 H 12 O 6(aq) + 6 O 2(g)
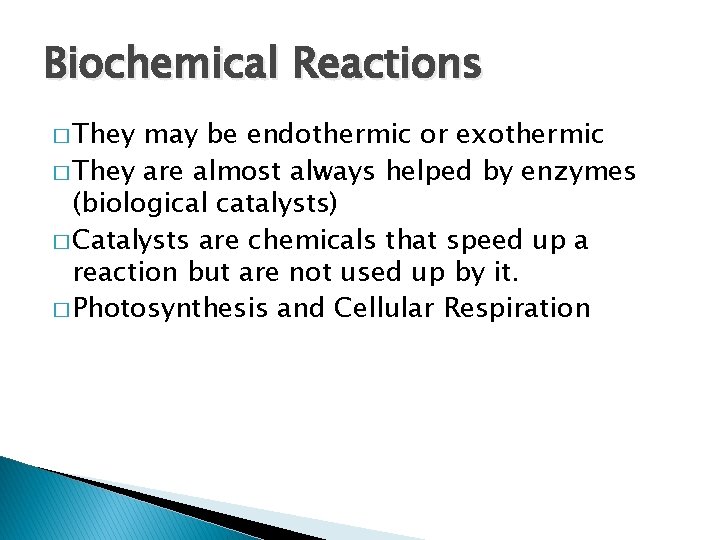
Biochemical Reactions � They may be endothermic or exothermic � They are almost always helped by enzymes (biological catalysts) � Catalysts are chemicals that speed up a reaction but are not used up by it. � Photosynthesis and Cellular Respiration

Law of Conservation of Mass � Developed by Antoine Lavoisier � Total mass of the reactants equals the total mass of the products � Using this, we can deduce that: ◦ ** total # of atoms present before a reaction equals the total # of atoms after a reaction
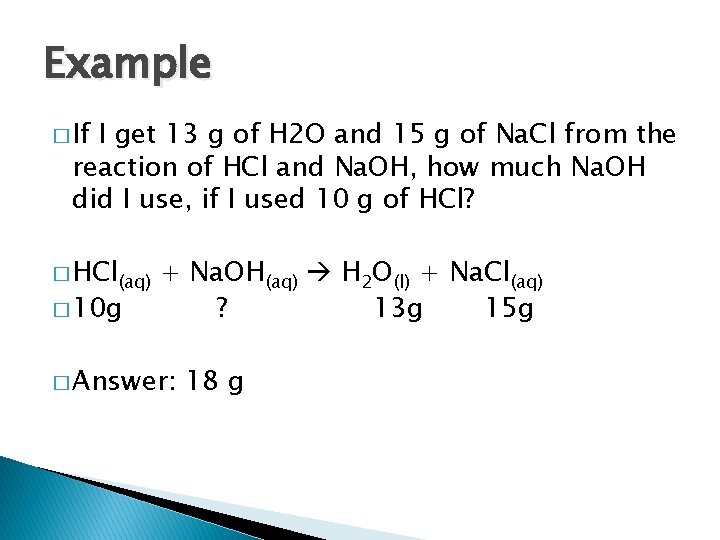
Example � If I get 13 g of H 2 O and 15 g of Na. Cl from the reaction of HCl and Na. OH, how much Na. OH did I use, if I used 10 g of HCl? � HCl(aq) � 10 g + Na. OH(aq) H 2 O(l) + Na. Cl(aq) ? 13 g 15 g � Answer: 18 g
- Slides: 14