Chemical Calculations Lesson 1 Mass Moles and Molar














- Slides: 18

Chemical Calculations Lesson # 1 Mass, Moles, and Molar Mass

The Mole o It is important in chemistry to be able to measure the amount of a substance, whether it be in industrial applications (large scale) or in microscopic applications (tiny scale). o The mole is the unit for the amount of a substance (symbol n). o The term “mole” is just a name for a particular number of particles, like how the word “dozen” is equivalent to 12, and a “pair” means 2. o A mole represents the number 6. 02 x 1023 – this number is called Avogadro’s number (NA), because of the contribution of research of scientist Amedeo Avogadro.

The Mole (continued) o When we say we have one mole of carbon, we in fact have 6. 02 x 1023 atoms of carbon. o The reason that we talk in “moles” in the first place, is because we cannot simply work with one atom – it is too tiny. Avogadro’s number is a more reasonable amount of atoms that can be seen, manipulated, and measured on a scale. o If we want to calculate how many moles we have of a substance based on the number of atoms, or vice versa, Avogadro’s number gives us a conversion factor: 1 mole = 6. 02 x 1023 atoms or molecules of a substance.

Moles (continued) o Symbol used for the mole: n o SI unit of the mole: mol

Example 1 – How many atoms are their in two moles of Helium?

Example 2 – How many moles are in 8. 25 x 1023 molecules of H 2 O?

Summary Moles to Atoms: Multiply by NA n Atoms to Moles: Divide by NA n

Molar Mass o Knowing amounts in moles can be helpful at times, but when you are working in the lab, it’s not enough to know that a compound A 2 B 3 C 4 has 2 moles of A, 3 moles of B, and 4 moles of C – you need to know how much it weighs to be able to add it in to your chemical! o Conveniently enough, the mass, in grams, of one mole of atoms or molecules is called the molar mass!

Molar Mass (continued) o Symbol used for molar mass: M o SI unit of molar mass: g/mol o This means that molar mass is equal to the mass (in grams) divided by its amount (in moles), or: o Molar mass = (M = m) n o This value can be found on your periodic table – you called it “atomic mass” in grade 9 and 10.

Example 1 – what is the mass of water?

Example 2 – What is the mass of PCl 5?

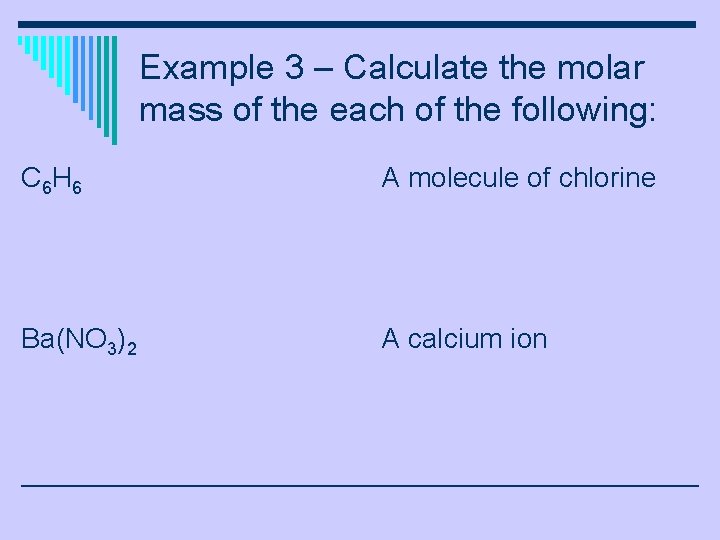
Example 3 – Calculate the molar mass of the each of the following: C 6 H 6 A molecule of chlorine Ba(NO 3)2 A calcium ion

Example 1 – calculate the mass of 0. 50 mol of sulfur

Example 2 – calculate the mass of 2. 50 mol of iron

Example 3 – calculate the amount of substance (number of moles) in 5. 20 g of hydrogen gas (H 2)

Example 4 – calculate the amount of substance (number of moles) in 8. 75 g of iron (III) chloride (Fe. Cl 3)

Summary o Mass to Moles: Divide by Molar Mass o Moles to Mass: Multiply by Molar Mass

Videos o Mole Explanation o Molar Eclipse of the Heart