Chemical Calculations Formula Masses Moles and Chemical Equations










- Slides: 50

Chemical Calculations: Formula Masses, Moles, and Chemical Equations. CHAPTER 6

CHAPTER 6 6. 1 Formula masses 6. 2 The mole: A counting unit for chemists 6. 3 The mass of a mole 6. 4 Chemical formulas and the mole concept 6. 5 The mole and chemical calculations 6. 6 Writing and balancing chemical equations 6. 7 Chemical equations and the mole concept 6. 8 Chemical calculations using chemical equations 6. 9 Yields: Theoretical, actual, and percent

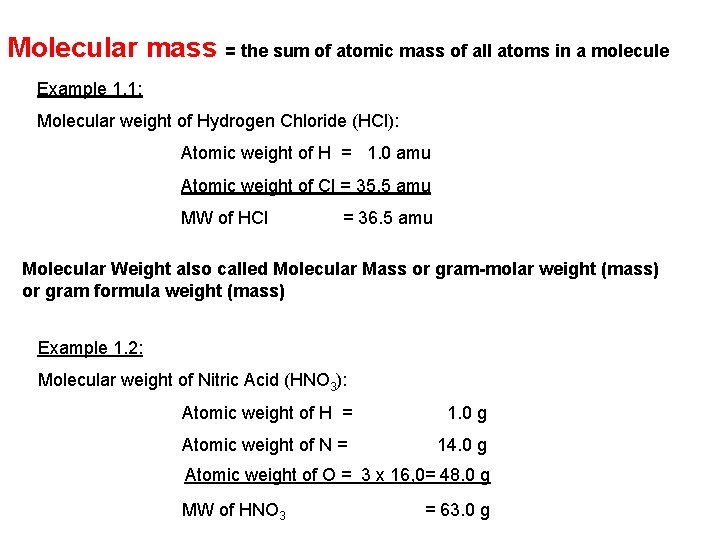
Chapter 6 Chemical Calculations: Formula Masses, Moles, & Chemical Equations Atomic Mass = the weighted average of masses of all naturally-occurring isotopes of an element Formula Mass = the sum of the atomic masses of all the atoms represented in the chemical formula Calculate the Formula Mass: Sn. F 2 Formula Mass of Sn. F 2 = 156. 71 amu Al(OH)3 Formula Mass of Al(OH)3 = 78. 0036 g/mol 118. 71+ (2 × 19. 00)

Calculating Molar Mass of a Compound H 2 O - C 2 H 6 O (NH 4)2 Cl The masses of the elements on the PERIODIC TABLE are often referred to as atomic weights.

Molecular mass = the sum of atomic mass of all atoms in a molecule Example 1. 1: Molecular weight of Hydrogen Chloride (HCl): Atomic weight of H = 1. 0 amu Atomic weight of Cl = 35. 5 amu MW of HCl = 36. 5 amu Molecular Weight also called Molecular Mass or gram-molar weight (mass) or gram formula weight (mass) Example 1. 2: Molecular weight of Nitric Acid (HNO 3): Atomic weight of H = 1. 0 g Atomic weight of N = 14. 0 g Atomic weight of O = 3 x 16, 0= 48. 0 g MW of HNO 3 = 63. 0 g

The Mole A chemistry counting unit! 1 Mole of an element = the atomic mass in grams

Mole = the amount of substance whose mass in grams is numerically equal to its molar or formula weight (mass) Mole = is the molecular weight (mass) of the compound in grams Example 1. 3: Molecular weight of Hydrogen Chloride (HCl): Atomic weight of H = 1. 0 g Atomic weight of Cl = 35. 5 g MW of HCl = 36. 5 g/mol 1 mole of HCl = 36. 5 g 2 moles of HCl = 2 x 36. 5 g= 73 g etc. Example 1. 4: Molecular weight of H 2 CO 3: Atomic weight of H = 2 x 1. 0 g = 2. 0 g Atomic weight of C = 12. 0 g Atomic weight of O = 3 x 16. 0 g = 48. 0 g 1 mole of H 2 CO 3 = 62. 0 g/mol

What does a mole look like? Copper Cu Carbon C Water H 2 O Sugar C 6 H 12 O 6 Salt Na. Cl

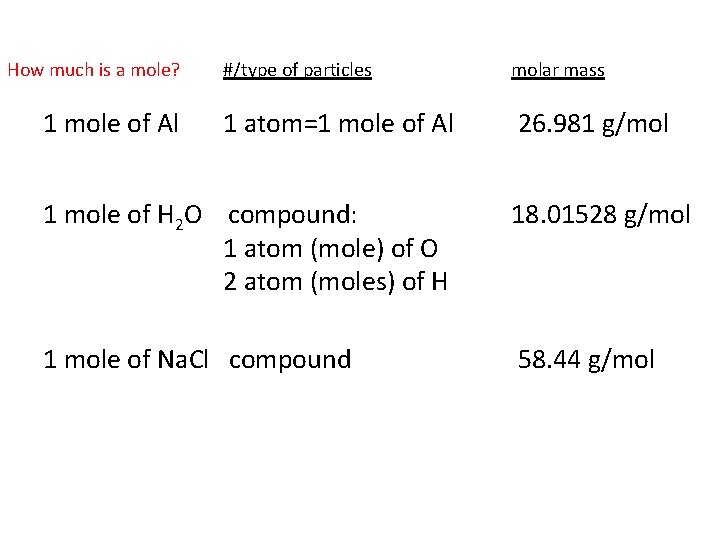
How much is a mole? 1 mole of Al #/type of particles molar mass 1 atom=1 mole of Al 26. 981 g/mol 1 mole of H 2 O compound: 1 atom (mole) of O 2 atom (moles) of H 18. 01528 g/mol 1 mole of Na. Cl compound 58. 44 g/mol

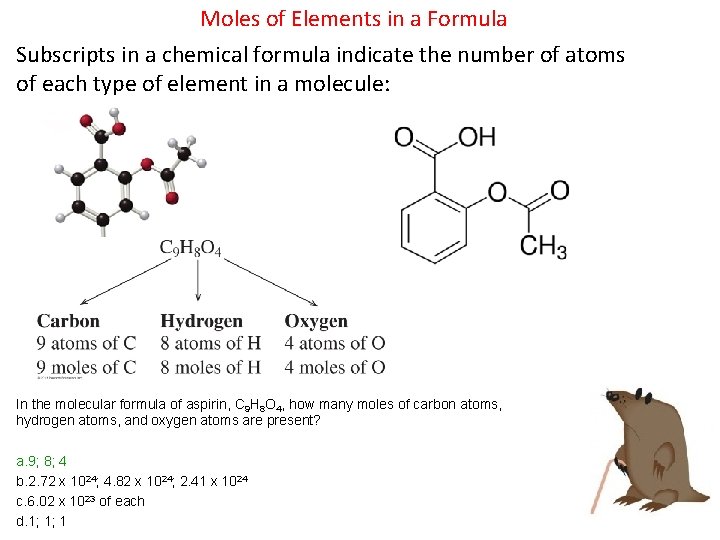
Moles of Elements in a Formula Subscripts in a chemical formula indicate the number of atoms of each type of element in a molecule: In the molecular formula of aspirin, C 9 H 8 O 4, how many moles of carbon atoms, hydrogen atoms, and oxygen atoms are present? a. 9; 8; 4 b. 2. 72 x 1024; 4. 82 x 1024; 2. 41 x 1024 c. 6. 02 x 1023 of each d. 1; 1; 1

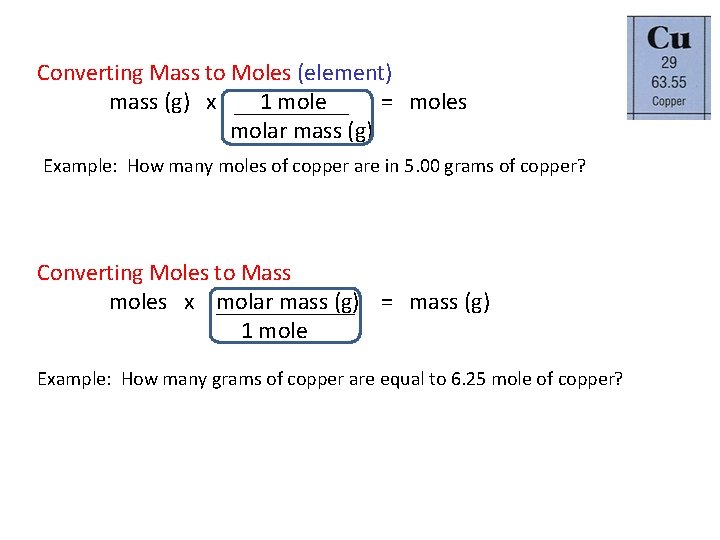
Converting Mass to Moles (element) mass (g) x 1 mole = moles molar mass (g) Example: How many moles of copper are in 5. 00 grams of copper? Converting Moles to Mass moles x molar mass (g) = mass (g) 1 mole Example: How many grams of copper are equal to 6. 25 mole of copper?

Avogadro’s Number (NA) – the number of units in 1 mole of anything 6. 022 x 1023 Avogadro’s Number (NA) – is the number of atoms in 1 gram atomic weight of element Individual atoms will differ in size and weight from element to element, but the number of atoms in one gram atomic weight of any element has been experimentally determined to be 6. 022 x 10 23 Example 1. 4. 1 atom of sodium = 22. 98977 amu (or u) ≈ 23. 0 u So 23. 0 g of sodium contains 6. 022 x 1023 atoms There is relationship between atomic mass unit (amu) and the gram. 6. 022 x 1023 amu/gram or Avogadro’s Number (NA) number of ions, particles or moles is referred to as 1 mole of the substance. Example 1. 5. 1 mole of HCL = 6. 022 x 1023 HCl molecules = 36. 5 g HCl


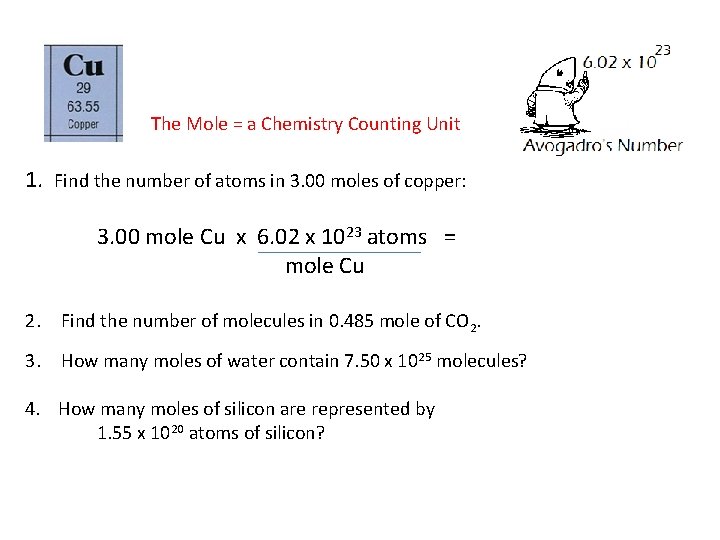
The Mole = a Chemistry Counting Unit 1. Find the number of atoms in 3. 00 moles of copper: 3. 00 mole Cu x 6. 02 x 1023 atoms = mole Cu 2. Find the number of molecules in 0. 485 mole of CO 2. 3. How many moles of water contain 7. 50 x 1025 molecules? 4. How many moles of silicon are represented by 1. 55 x 1020 atoms of silicon?

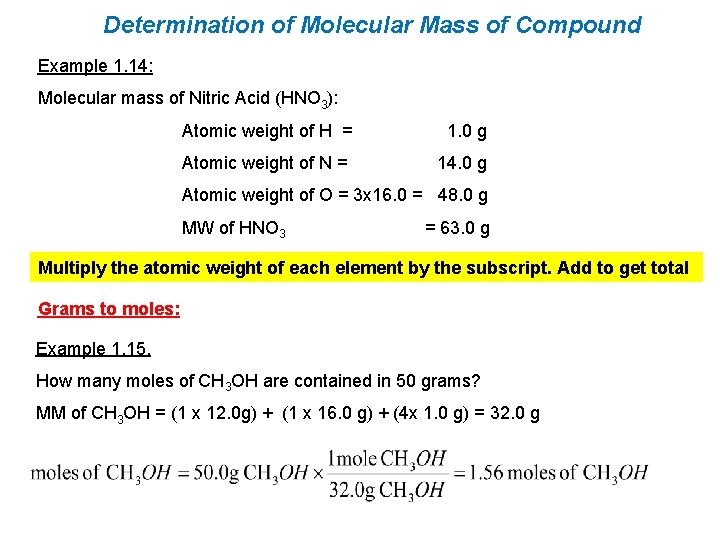
Determination of Molecular Mass of Compound Example 1. 14: Molecular mass of Nitric Acid (HNO 3): Atomic weight of H = 1. 0 g Atomic weight of N = 14. 0 g Atomic weight of O = 3 x 16. 0 = 48. 0 g MW of HNO 3 = 63. 0 g Multiply the atomic weight of each element by the subscript. Add to get total Grams to moles: Example 1. 15. How many moles of CH 3 OH are contained in 50 grams? MM of CH 3 OH = (1 x 12. 0 g) + (1 x 16. 0 g) + (4 x 1. 0 g) = 32. 0 g

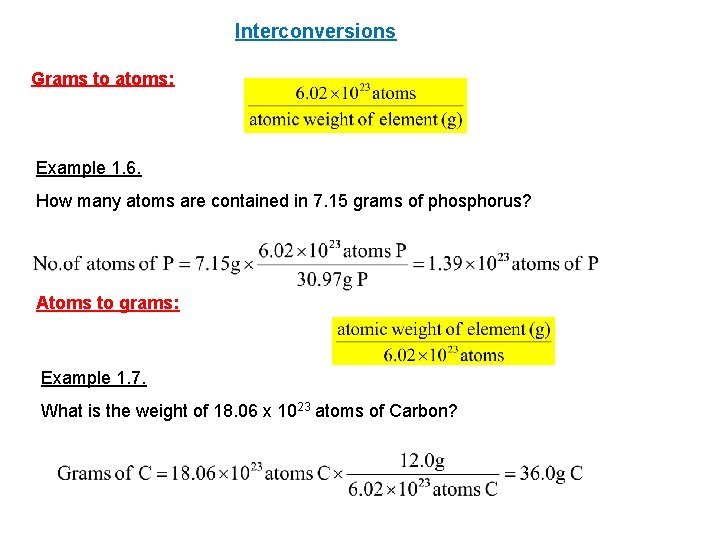
Interconversions Grams to atoms: Example 1. 6. How many atoms are contained in 7. 15 grams of phosphorus? Atoms to grams: Example 1. 7. What is the weight of 18. 06 x 1023 atoms of Carbon?

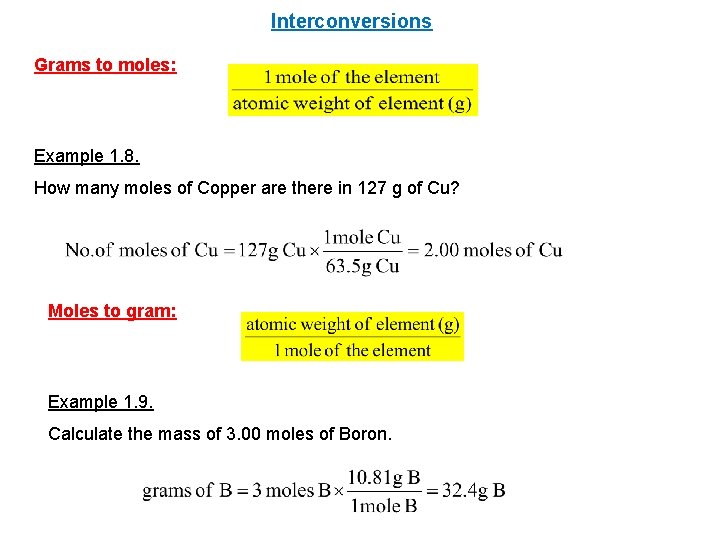
Interconversions Grams to moles: Example 1. 8. How many moles of Copper are there in 127 g of Cu? Moles to gram: Example 1. 9. Calculate the mass of 3. 00 moles of Boron.

Interconversions Atoms to moles: Example 1. 10. 2. 50 x 1024 atoms of silver is how many moles of silver? Moles to atoms: Example 1. 11. How many atoms of Lithium are contained in 6. 10 moles of Lithium?

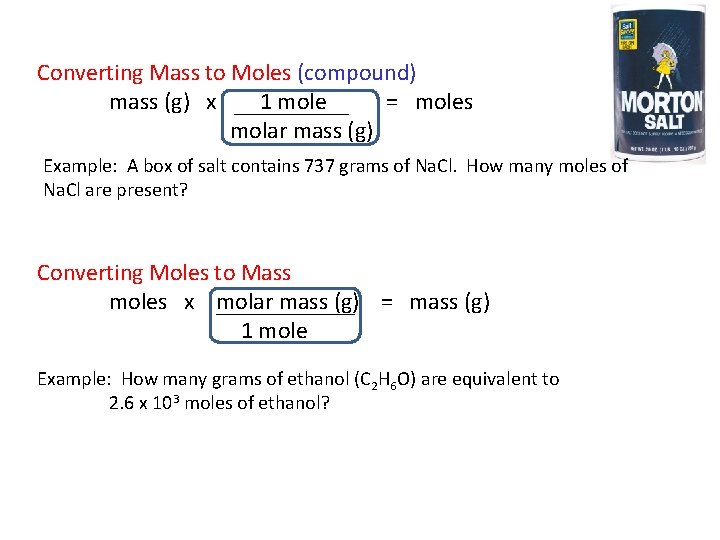
Converting Mass to Moles (compound) mass (g) x 1 mole = moles molar mass (g) Example: A box of salt contains 737 grams of Na. Cl. How many moles of Na. Cl are present? Converting Moles to Mass moles x molar mass (g) = mass (g) 1 mole Example: How many grams of ethanol (C 2 H 6 O) are equivalent to 2. 6 x 103 moles of ethanol?

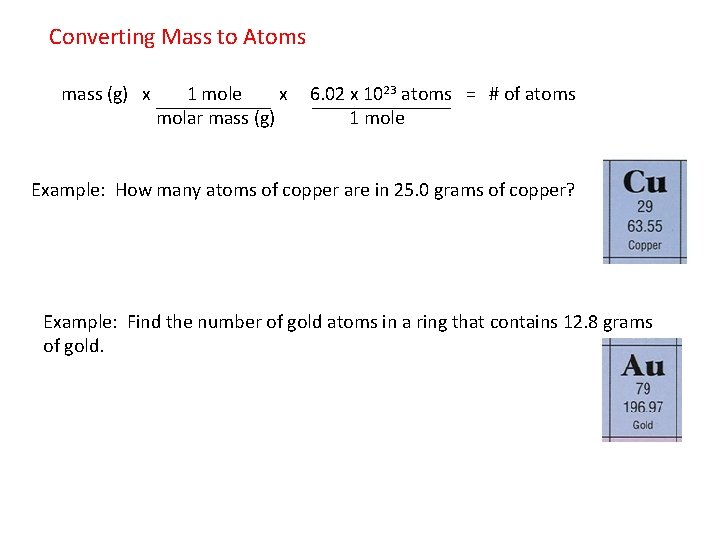
Converting Mass to Atoms mass (g) x 1 mole x 6. 02 x 1023 atoms = # of atoms molar mass (g) 1 mole Example: How many atoms of copper are in 25. 0 grams of copper? Example: Find the number of gold atoms in a ring that contains 12. 8 grams of gold.

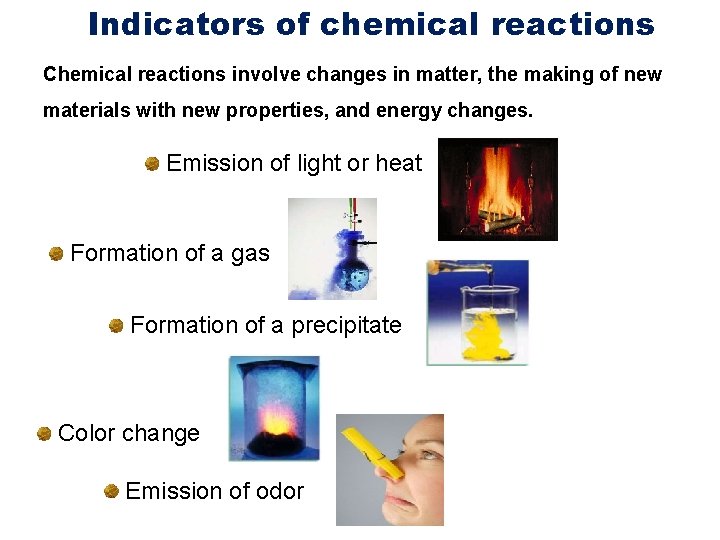
Indicators of chemical reactions Chemical reactions involve changes in matter, the making of new materials with new properties, and energy changes. Emission of light or heat Formation of a gas Formation of a precipitate Color change Emission of odor

All chemical reactions: • Reactants - the substances you start with • Products- the substances you end up with • The reactants turn into the products. Reactants → Products

Symbols used in equations • (s) after the formula –solid Cu(s) • (g) after the formula –gas H 2 (g) • (l) after the formula -liquid H 2 O(l) • (aq) after the formula - dissolved in water, an aqueous solution. Ca. Cl 2 (aq)

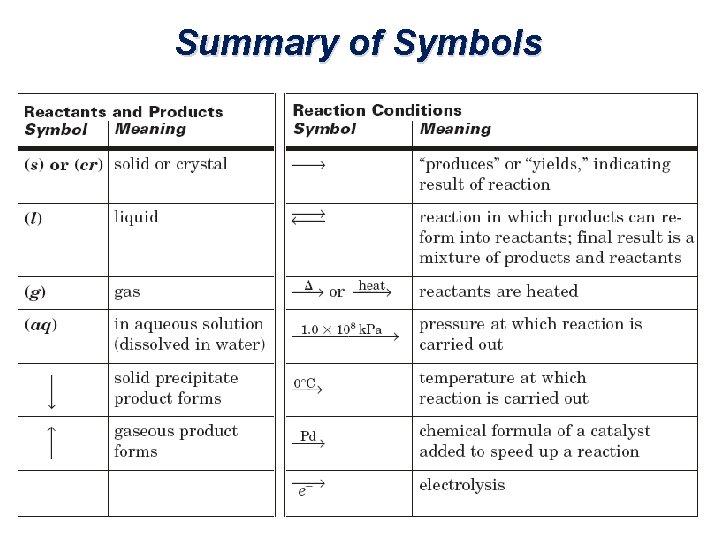
Summary of Symbols

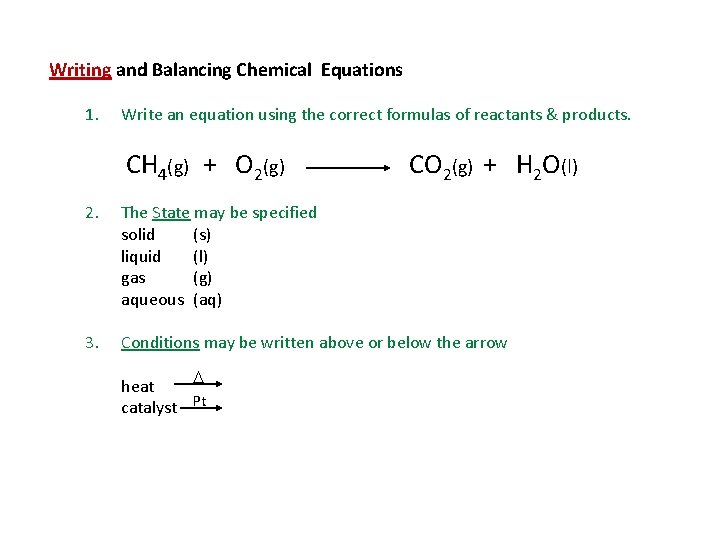
Writing and Balancing Chemical Equations 1. Write an equation using the correct formulas of reactants & products. CH 4(g) + O 2(g) CO 2(g) + H 2 O(l) 2. The State may be specified solid (s) liquid (l) gas (g) aqueous (aq) 3. Conditions may be written above or below the arrow heat catalyst Pt

What is a catalyst? • A substance that speeds up a reaction without being changed by the reaction. • Enzymes are biological or protein catalysts. Example: Fe 3 H 2 + N 2 → 2 NH 3 Haber process crude oil → gasoline Catalytic Cracking zeolite sulfuric acid alcohol + acid → ester Fischer esterification

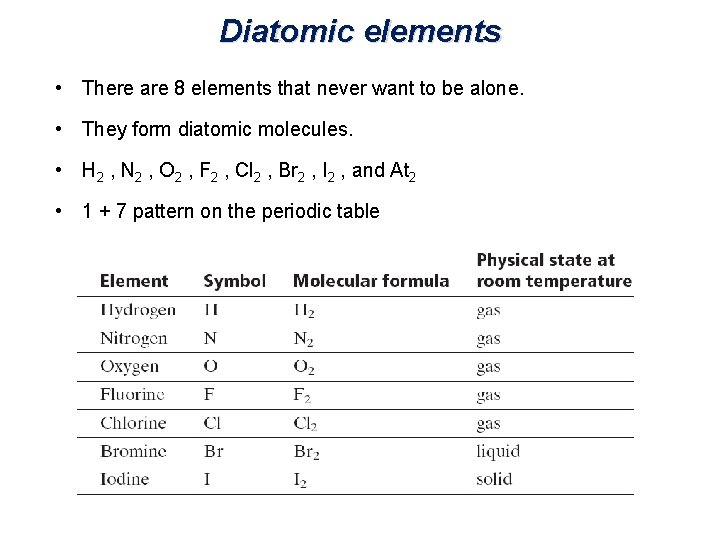
Diatomic elements • There are 8 elements that never want to be alone. • They form diatomic molecules. • H 2 , N 2 , O 2 , F 2 , Cl 2 , Br 2 , I 2 , and At 2 • 1 + 7 pattern on the periodic table

Chemical Equations Because of the principle of the conservation of matter, an equation must be balanced. Antoine Lavoisier (1743 – 1794) It must have the same number of atoms of the same kind on both sides.

In a reaction, atoms are neither created or destroyed… So, chemical equation must have an equal number of each type of atom on both side s of reaction arrow. Start by balancing those elements that occur in only one compound on each side of the equation. You can only change the COEFFICIENTS, while balancing the chemical reaction. NEVER subscripts!

Balancing Equations – When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but – you may not change the subscripts. • Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent)

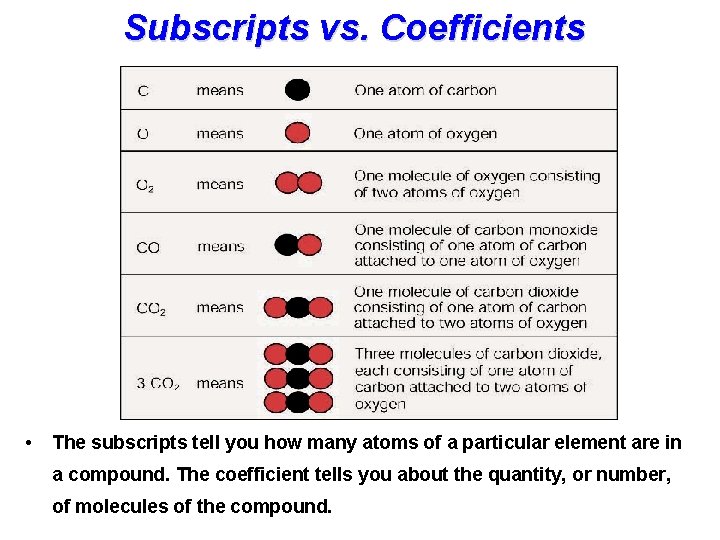
Subscripts vs. Coefficients • The subscripts tell you how many atoms of a particular element are in a compound. The coefficient tells you about the quantity, or number, of molecules of the compound.

Steps to Balancing Equations There are four basic steps to balancing a chemical equation. 1. Write the correct formula for the reactants and the products. DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. And most importantly, once you write them correctly DO NOT CHANGE THE FORMULAS! 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. (reduced)

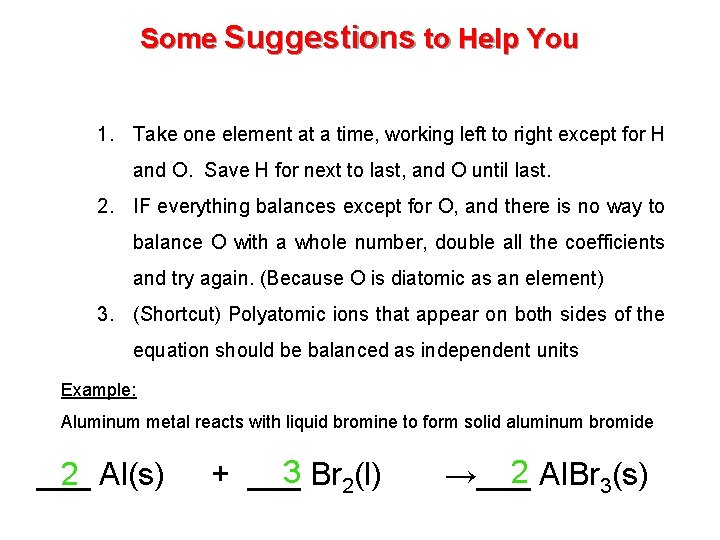
Some Suggestions to Help You 1. Take one element at a time, working left to right except for H and O. Save H for next to last, and O until last. 2. IF everything balances except for O, and there is no way to balance O with a whole number, double all the coefficients and try again. (Because O is diatomic as an element) 3. (Shortcut) Polyatomic ions that appear on both sides of the equation should be balanced as independent units Example: Aluminum metal reacts with liquid bromine to form solid aluminum bromide ___ Al(s) 2 3 2(l) + ___ Br 2 →___ Al. Br 3(s)

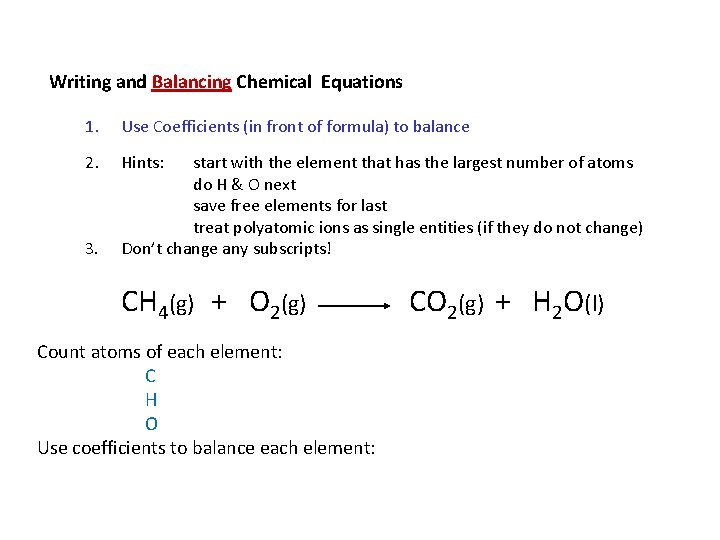
Writing and Balancing Chemical Equations 1. Use Coefficients (in front of formula) to balance 2. Hints: 3. start with the element that has the largest number of atoms do H & O next save free elements for last treat polyatomic ions as single entities (if they do not change) Don’t change any subscripts! CH 4(g) + O 2(g) Count atoms of each element: C H O Use coefficients to balance each element: CO 2(g) + H 2 O(l)

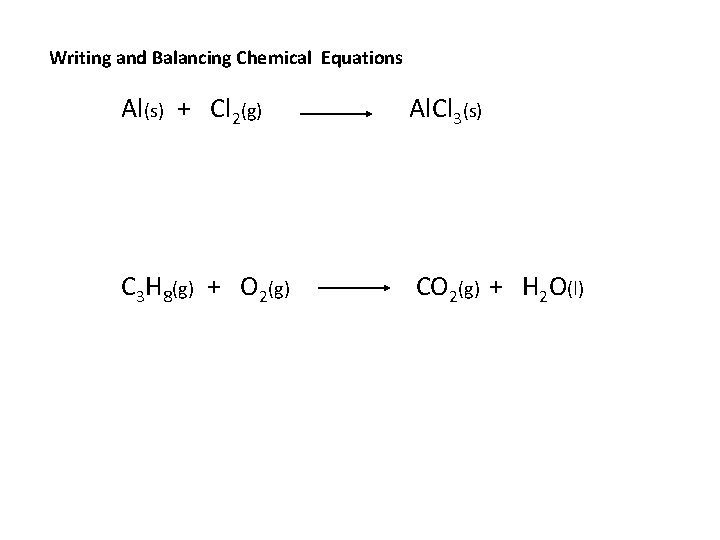
Writing and Balancing Chemical Equations Al(s) + Cl 2(g) Al. Cl 3(s) C 3 H 8(g) + O 2(g) CO 2(g) + H 2 O(l)

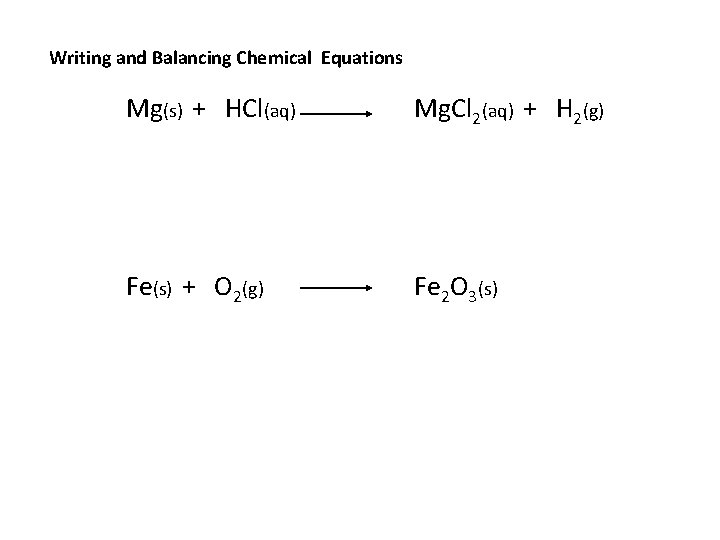
Writing and Balancing Chemical Equations Mg(s) + HCl(aq) Mg. Cl 2(aq) + H 2(g) Fe(s) + O 2(g) Fe 2 O 3(s)

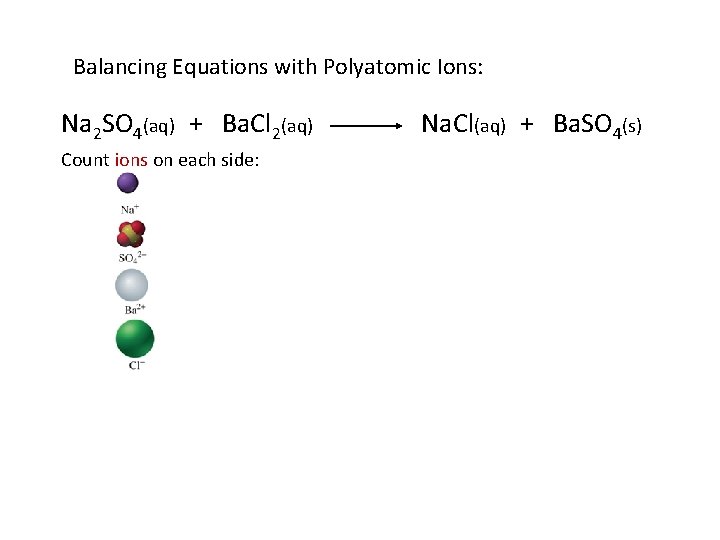
Balancing Equations with Polyatomic Ions: Na 2 SO 4(aq) + Ba. Cl 2(aq) Count ions on each side: Na. Cl(aq) + Ba. SO 4(s)

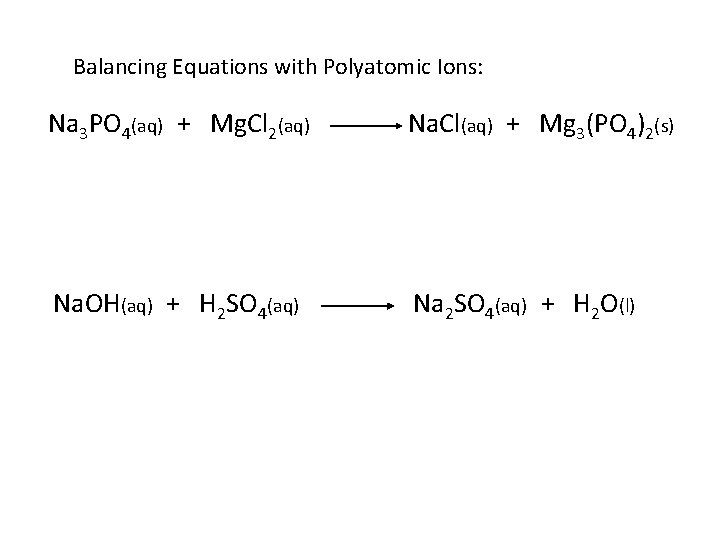
Balancing Equations with Polyatomic Ions: Na 3 PO 4(aq) + Mg. Cl 2(aq) Na. Cl(aq) + Mg 3(PO 4)2(s) Na. OH(aq) + H 2 SO 4(aq) Na 2 SO 4(aq) + H 2 O(l)

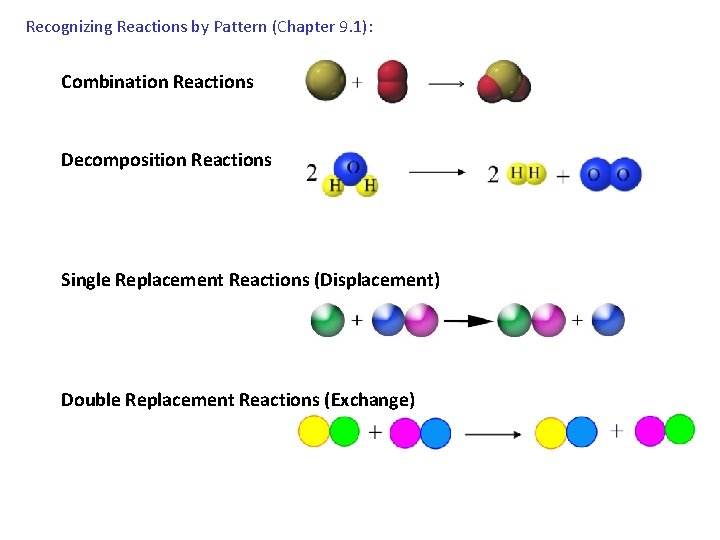
Recognizing Reactions by Pattern (Chapter 9. 1): Combination Reactions Decomposition Reactions Single Replacement Reactions (Displacement) Double Replacement Reactions (Exchange)

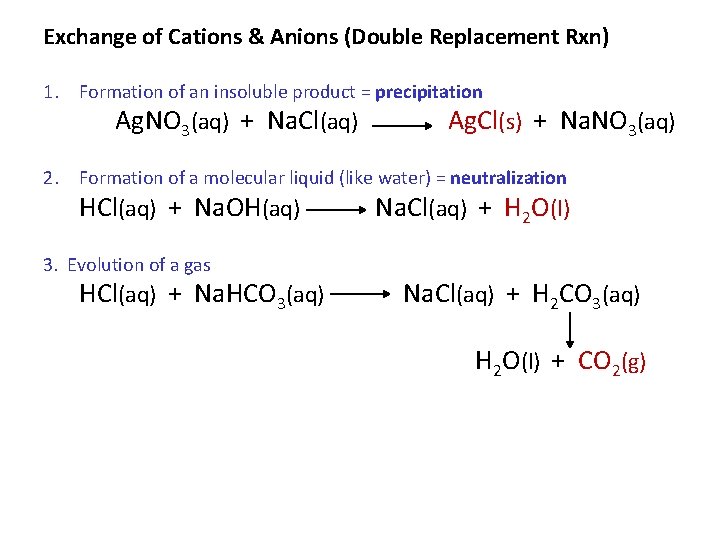
Exchange of Cations & Anions (Double Replacement Rxn) 1. Formation of an insoluble product = precipitation Ag. NO 3(aq) + Na. Cl(aq) Ag. Cl(s) + Na. NO 3(aq) 2. Formation of a molecular liquid (like water) = neutralization HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) 3. Evolution of a gas HCl(aq) + Na. HCO 3(aq) Na. Cl(aq) + H 2 CO 3(aq) H 2 O(l) + CO 2(g)

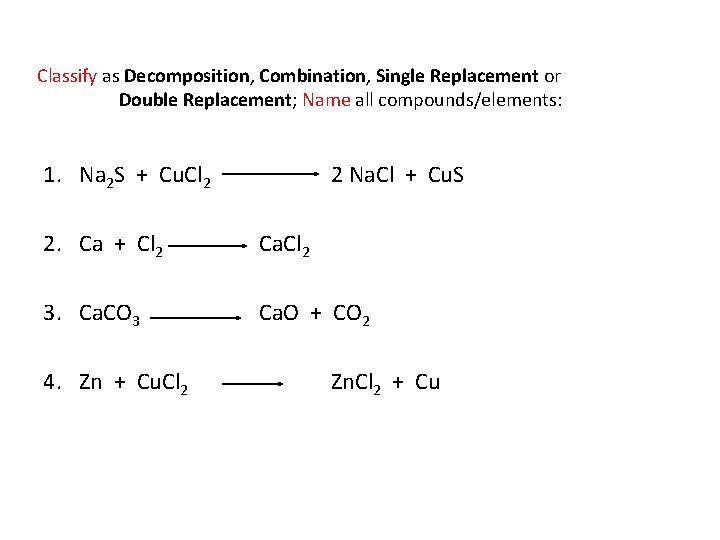
Classify as Decomposition, Combination, Single Replacement or Double Replacement; Name all compounds/elements: 1. Na 2 S + Cu. Cl 2 2 Na. Cl + Cu. S 2. Ca + Cl 2 Ca. Cl 2 3. Ca. CO 3 Ca. O + CO 2 4. Zn + Cu. Cl 2 Zn. Cl 2 + Cu

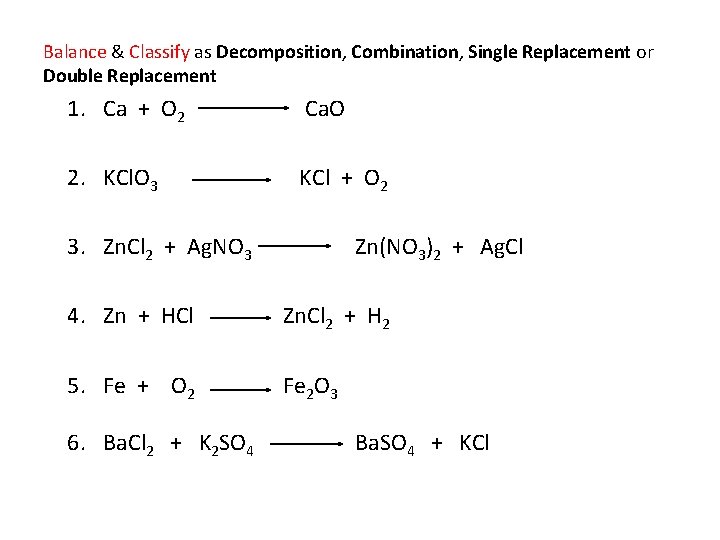
Balance & Classify as Decomposition, Combination, Single Replacement or Double Replacement 1. Ca + O 2 Ca. O 2. KCl. O 3 KCl + O 2 3. Zn. Cl 2 + Ag. NO 3 Zn(NO 3)2 + Ag. Cl 4. Zn + HCl Zn. Cl 2 + H 2 5. Fe + O 2 Fe 2 O 3 6. Ba. Cl 2 + K 2 SO 4 Ba. SO 4 + KCl

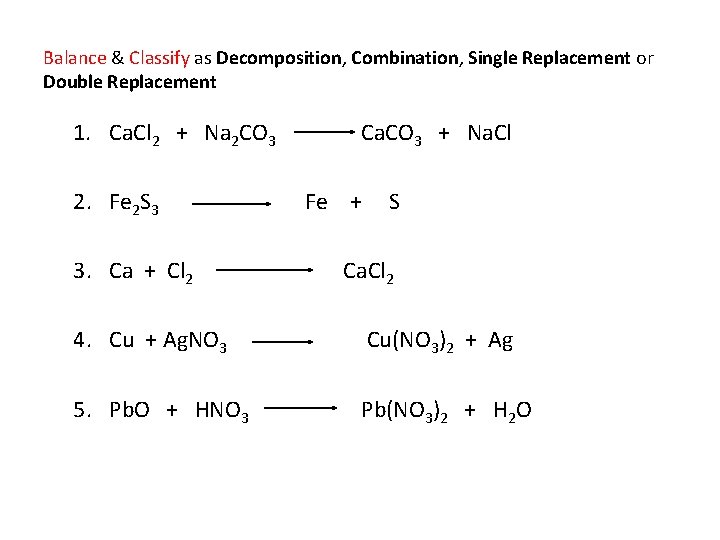
Balance & Classify as Decomposition, Combination, Single Replacement or Double Replacement 1. Ca. Cl 2 + Na 2 CO 3 Ca. CO 3 + Na. Cl 2. Fe 2 S 3 Fe + S 3. Ca + Cl 2 Ca. Cl 2 4. Cu + Ag. NO 3 Cu(NO 3)2 + Ag 5. Pb. O + HNO 3 Pb(NO 3)2 + H 2 O

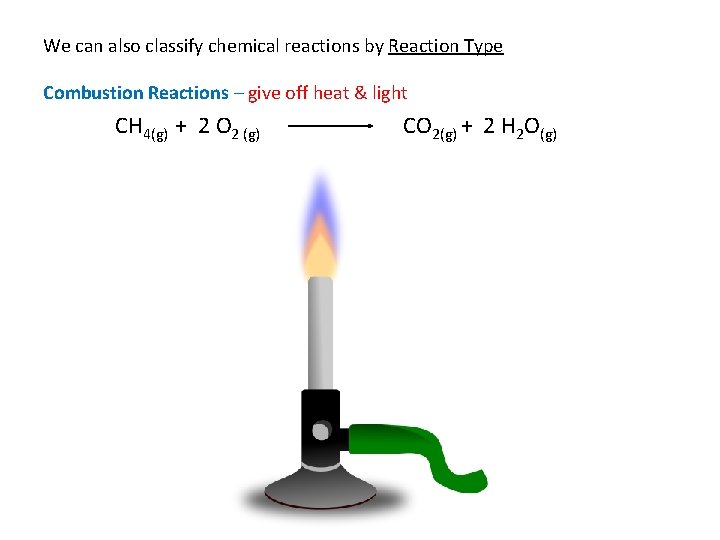
We can also classify chemical reactions by Reaction Type Combustion Reactions – give off heat & light CH 4(g) + 2 O 2 (g) CO 2(g) + 2 H 2 O(g)

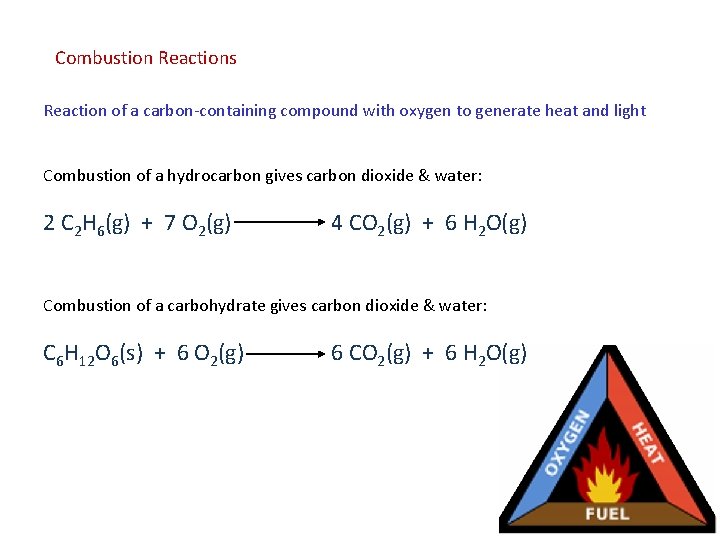
Combustion Reactions Reaction of a carbon-containing compound with oxygen to generate heat and light Combustion of a hydrocarbon gives carbon dioxide & water: 2 C 2 H 6(g) + 7 O 2(g) 4 CO 2(g) + 6 H 2 O(g) Combustion of a carbohydrate gives carbon dioxide & water: C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(g)

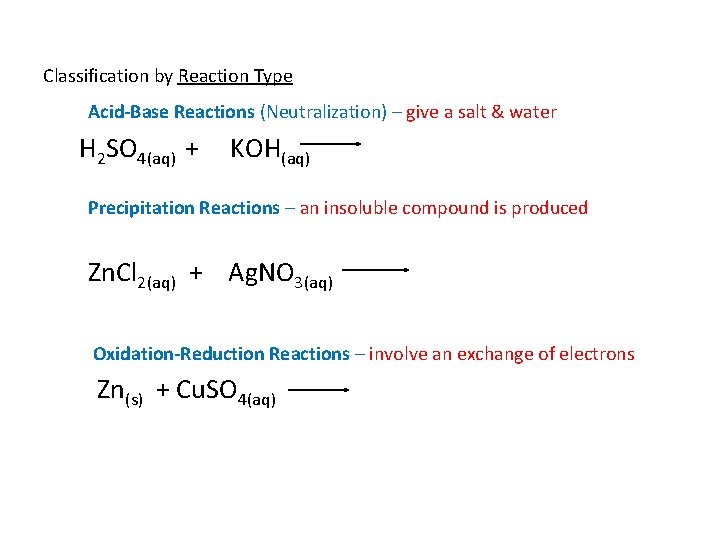
Classification by Reaction Type Acid-Base Reactions (Neutralization) – give a salt & water H 2 SO 4(aq) + KOH(aq) Precipitation Reactions – an insoluble compound is produced Zn. Cl 2(aq) + Ag. NO 3(aq) Oxidation-Reduction Reactions – involve an exchange of electrons Zn(s) + Cu. SO 4(aq)

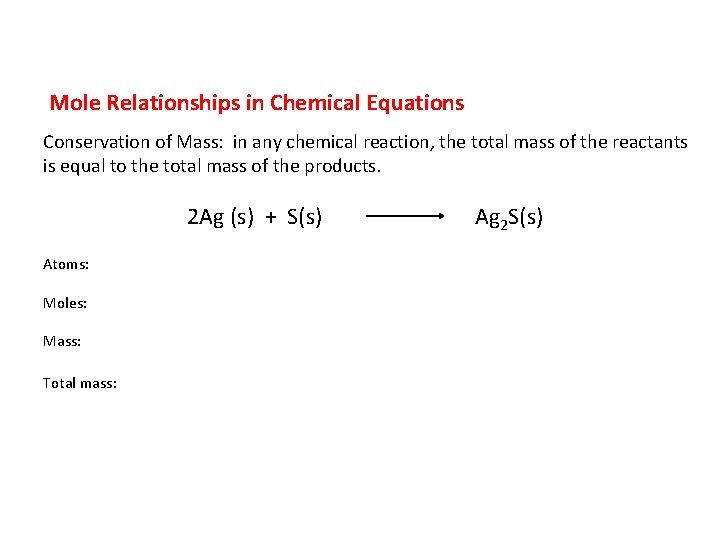
Mole Relationships in Chemical Equations Conservation of Mass: in any chemical reaction, the total mass of the reactants is equal to the total mass of the products. Atoms: Moles: Mass: Total mass: 2 Ag (s) + S(s) Ag 2 S(s)

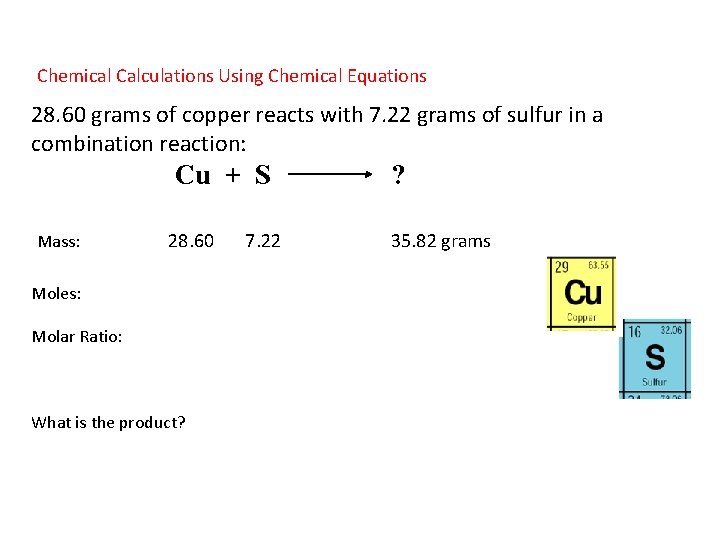
Chemical Calculations Using Chemical Equations 28. 60 grams of copper reacts with 7. 22 grams of sulfur in a combination reaction: Cu + S Mass: 28. 60 7. 22 Moles: Molar Ratio: What is the product? ? 35. 82 grams

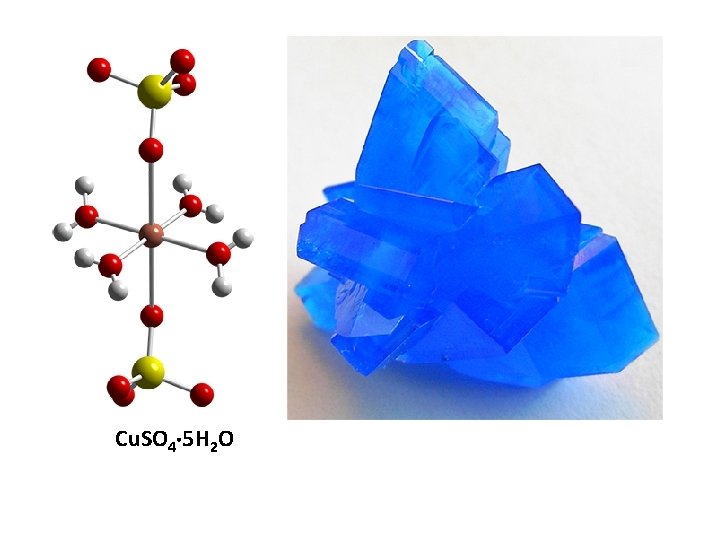
Cu. SO 4 5 H 2 O

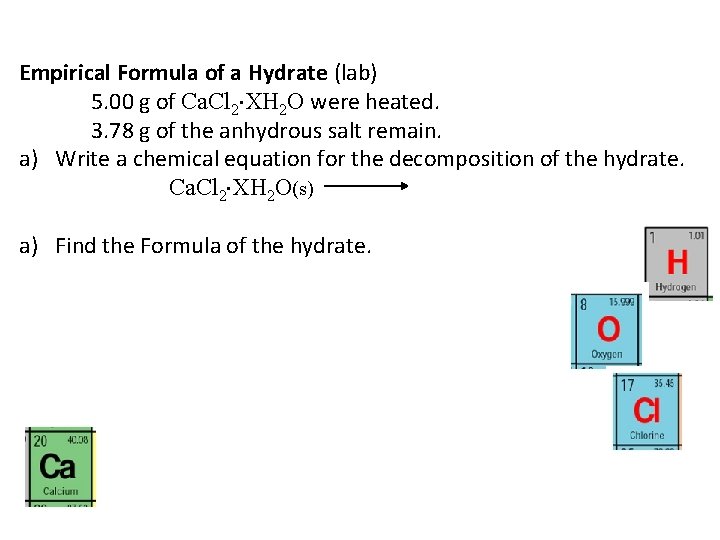
Empirical Formula of a Hydrate (lab) 5. 00 g of Ca. Cl 2 XH 2 O were heated. 3. 78 g of the anhydrous salt remain. a) Write a chemical equation for the decomposition of the hydrate. Ca. Cl 2 XH 2 O(s) a) Find the Formula of the hydrate.