CHEMICAL CALCULATIONS Chapter 9 Section 2 Balanced Equations
CHEMICAL CALCULATIONS Chapter 9 Section 2

Balanced Equations
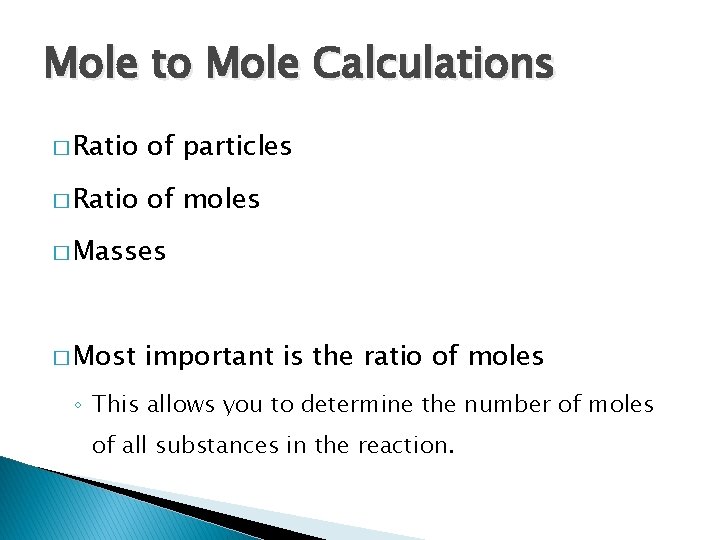
Mole to Mole Calculations � Ratio of particles � Ratio of moles � Masses � Most important is the ratio of moles ◦ This allows you to determine the number of moles of all substances in the reaction.
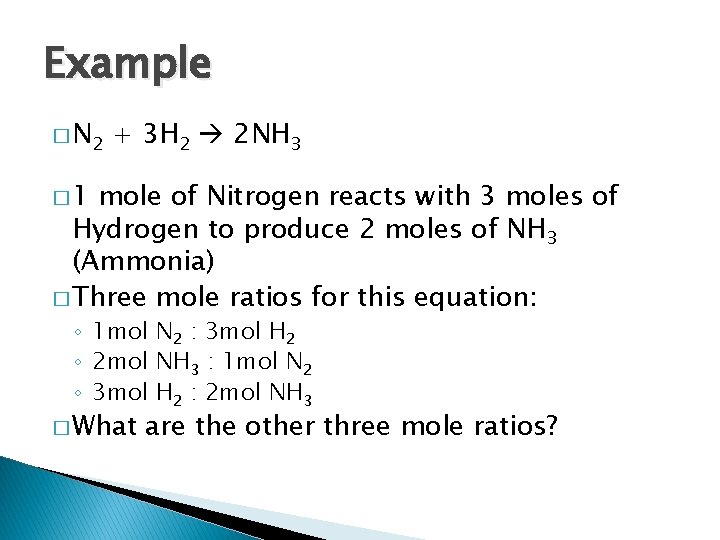
Example � N 2 + 3 H 2 2 NH 3 � 1 mole of Nitrogen reacts with 3 moles of Hydrogen to produce 2 moles of NH 3 (Ammonia) � Three mole ratios for this equation: ◦ 1 mol N 2 : 3 mol H 2 ◦ 2 mol NH 3 : 1 mol N 2 ◦ 3 mol H 2 : 2 mol NH 3 � What are the other three mole ratios?
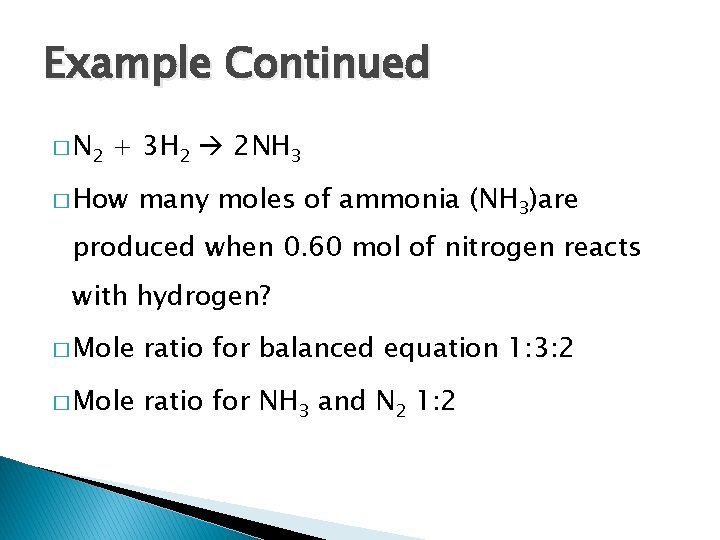
Example Continued � N 2 + 3 H 2 2 NH 3 � How many moles of ammonia (NH 3)are produced when 0. 60 mol of nitrogen reacts with hydrogen? � Mole ratio for balanced equation 1: 3: 2 � Mole ratio for NH 3 and N 2 1: 2

Example Continued � N 2 + 3 H 2 2 NH 3

Check for understanding � Page 244 � #9 and 10
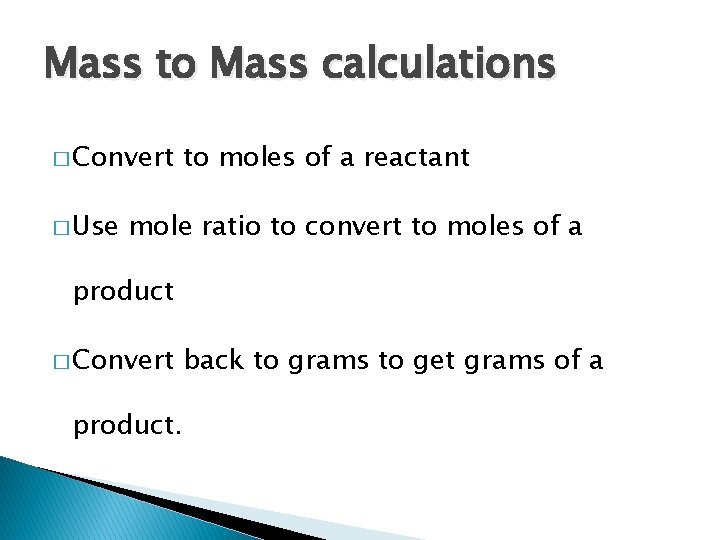
Mass to Mass calculations � Convert � Use to moles of a reactant mole ratio to convert to moles of a product � Convert back to grams to get grams of a product.
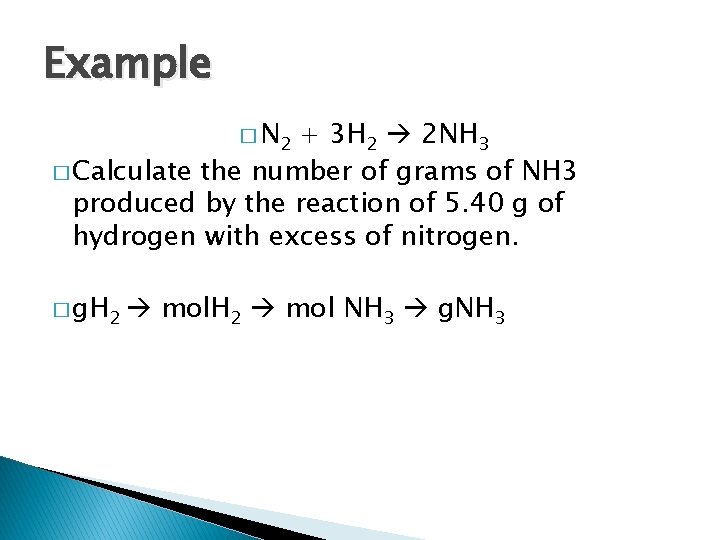
Example � N 2 + 3 H 2 2 NH 3 � Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with excess of nitrogen. � g. H 2 mol NH 3 g. NH 3
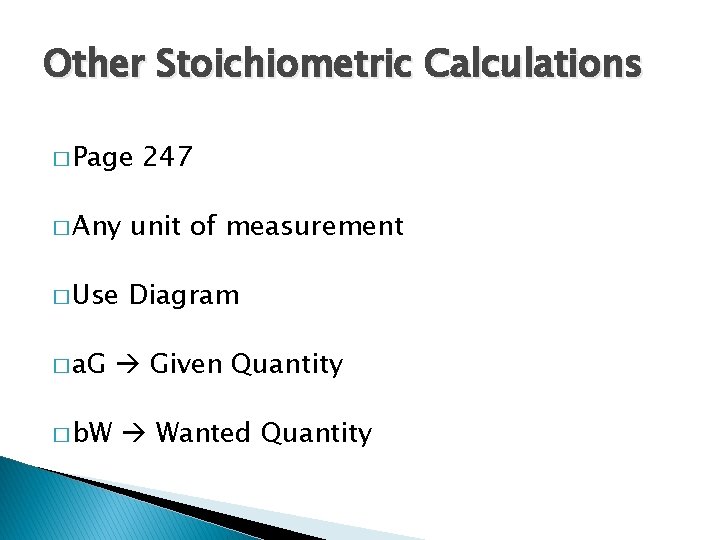
Other Stoichiometric Calculations � Page 247 � Any unit of measurement � Use Diagram � a. G � b. W Given Quantity Wanted Quantity
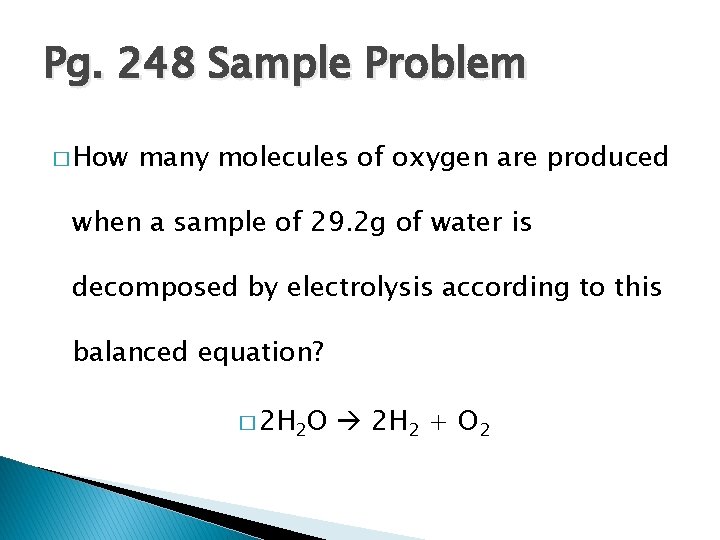
Pg. 248 Sample Problem � How many molecules of oxygen are produced when a sample of 29. 2 g of water is decomposed by electrolysis according to this balanced equation? � 2 H 2 O 2 H 2 + O 2

Practice
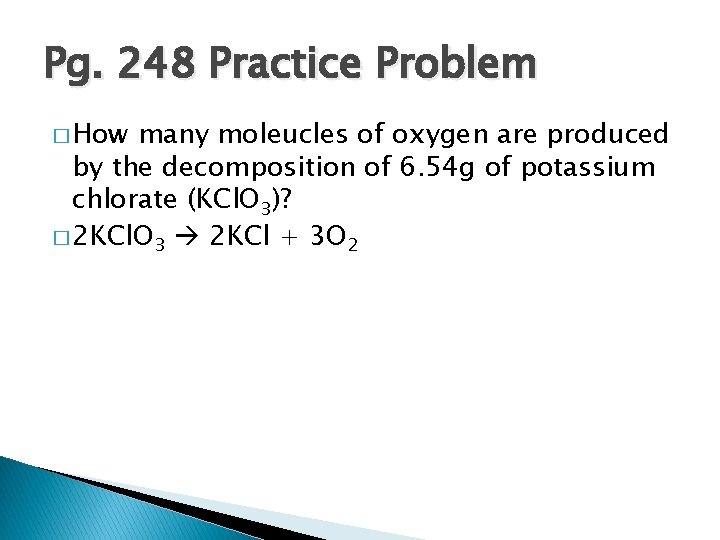
Pg. 248 Practice Problem � How many moleucles of oxygen are produced by the decomposition of 6. 54 g of potassium chlorate (KCl. O 3)? � 2 KCl. O 3 2 KCl + 3 O 2
- Slides: 13