Chemical Bonds Mutual attraction that binds atoms together

Chemical Bonds Mutual attraction that binds atoms together to form compounds
Types of Chemical Bonds • Ionic bond = bond that results from attraction between oppositely charged ions (transfer of electrons) • Covalent bond = bond resulting from the sharing of electrons
Metallic Bonds = metal atoms in a delocalized cloud of electrons • Remember: metals like to give up electrons, so no atom “wants” the free e-
Bonding happens on a spectrum • Bonds are rarely purely ionic or purely covalent • Remember Electronegativity (the ability of atoms to attract electrons) – Comparing the electronegativities of atoms involved in bond, can determine whether bond is ionic or covalent see page 161 PT for electronegativity values

Electronegativity difference above 1. 7 = ionic • Ex. Cs and F • Cs = 0. 7 • F = 3. 3
Electronegativity difference of 1. 7 or less = covalent • Polar-covalent (0. 3 -1. 7), – ex. H 2 O: H-2. 1, O-3. 5, diff = 1. 4 • Non-polar covalent (under 0. 3) – Ex. Bonds between atoms of the same element are always non-polar (purely) covalent, ex. H 2, O 2, N 2
Covalent Bonding Forms molecular compounds that consist of molecules
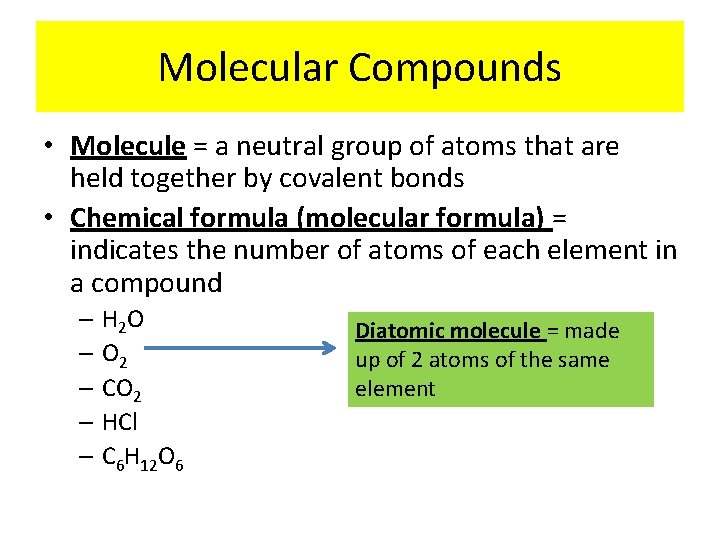
Molecular Compounds • Molecule = a neutral group of atoms that are held together by covalent bonds • Chemical formula (molecular formula) = indicates the number of atoms of each element in a compound – H 2 O – O 2 – CO 2 – HCl – C 6 H 12 O 6 Diatomic molecule = made up of 2 atoms of the same element
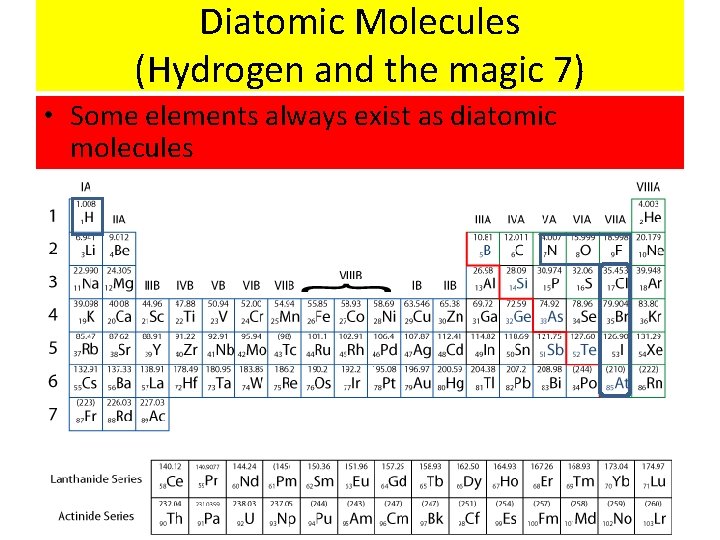
Diatomic Molecules (Hydrogen and the magic 7) • Some elements always exist as diatomic molecules
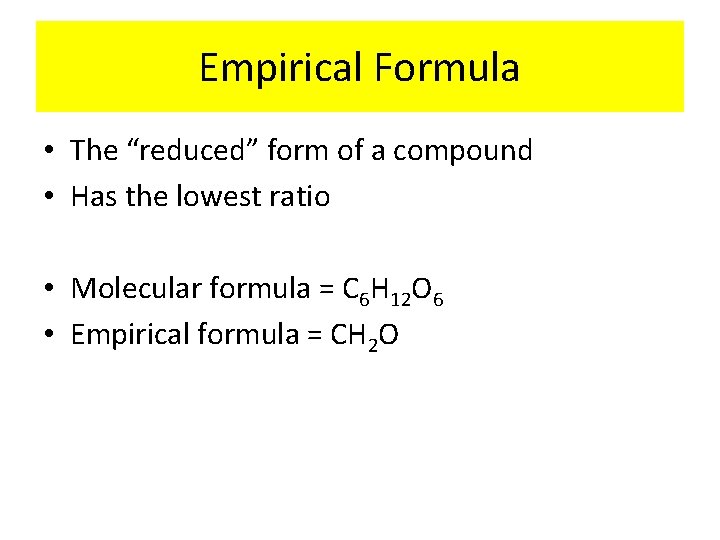
Empirical Formula • The “reduced” form of a compound • Has the lowest ratio • Molecular formula = C 6 H 12 O 6 • Empirical formula = CH 2 O
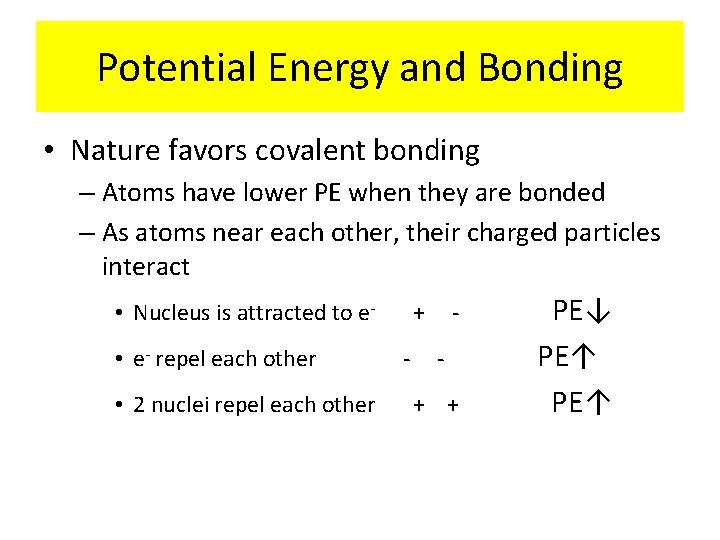
Potential Energy and Bonding • Nature favors covalent bonding – Atoms have lower PE when they are bonded – As atoms near each other, their charged particles interact • Nucleus is attracted to e • e- repel each other • 2 nuclei repel each other + - - + + PE↓ PE↑
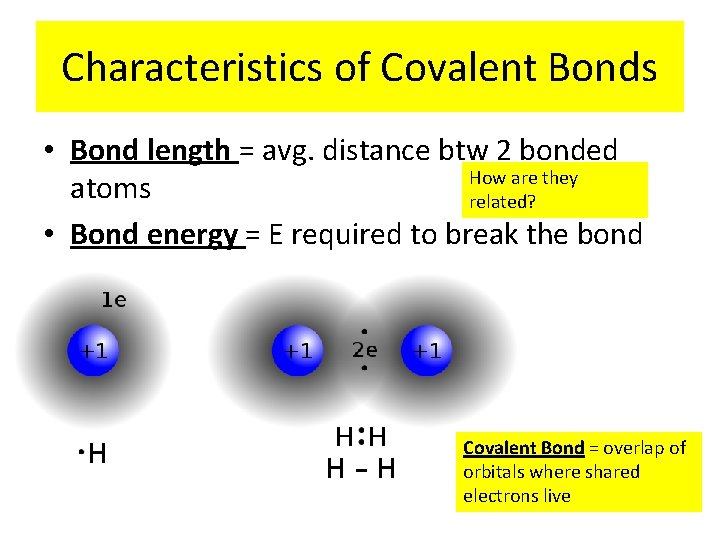
Characteristics of Covalent Bonds • Bond length = avg. distance btw 2 bonded How are they atoms related? • Bond energy = E required to break the bond Covalent Bond = overlap of orbitals where shared electrons live
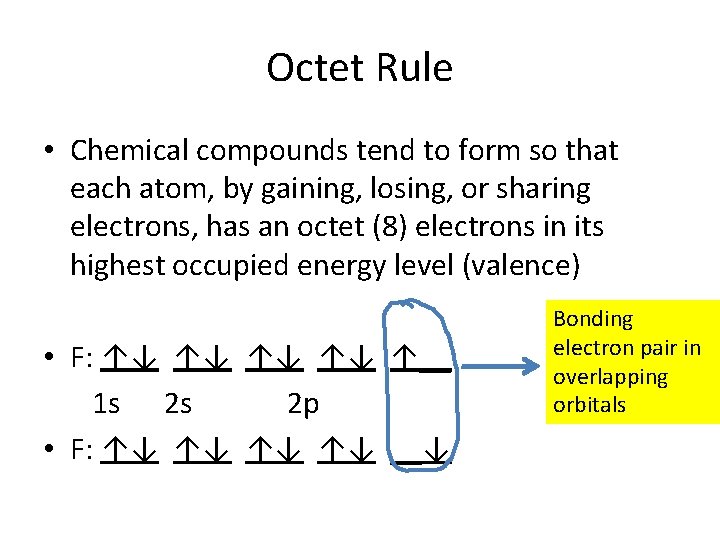
Octet Rule • Chemical compounds tend to form so that each atom, by gaining, losing, or sharing electrons, has an octet (8) electrons in its highest occupied energy level (valence) • F: ↑↓ ↑↓ ↑__ 1 s 2 s 2 p • F: ↑↓ ↑↓ __↓ Bonding electron pair in overlapping orbitals
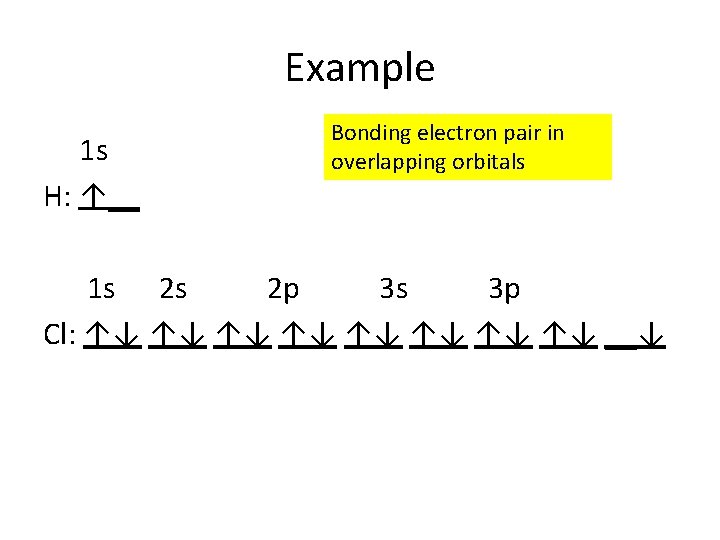
Example 1 s H: ↑__ Bonding electron pair in overlapping orbitals 1 s 2 s 2 p 3 s 3 p Cl: ↑↓ ↑↓ __↓
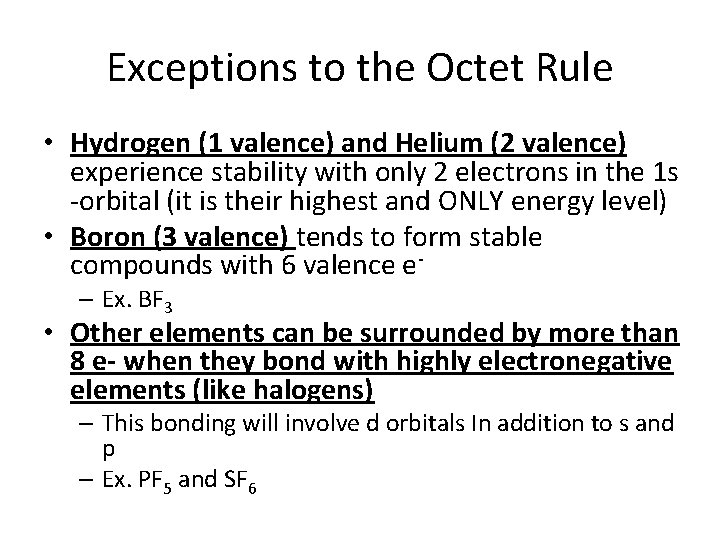
Exceptions to the Octet Rule • Hydrogen (1 valence) and Helium (2 valence) experience stability with only 2 electrons in the 1 s -orbital (it is their highest and ONLY energy level) • Boron (3 valence) tends to form stable compounds with 6 valence e– Ex. BF 3 • Other elements can be surrounded by more than 8 e- when they bond with highly electronegative elements (like halogens) – This bonding will involve d orbitals In addition to s and p – Ex. PF 5 and SF 6
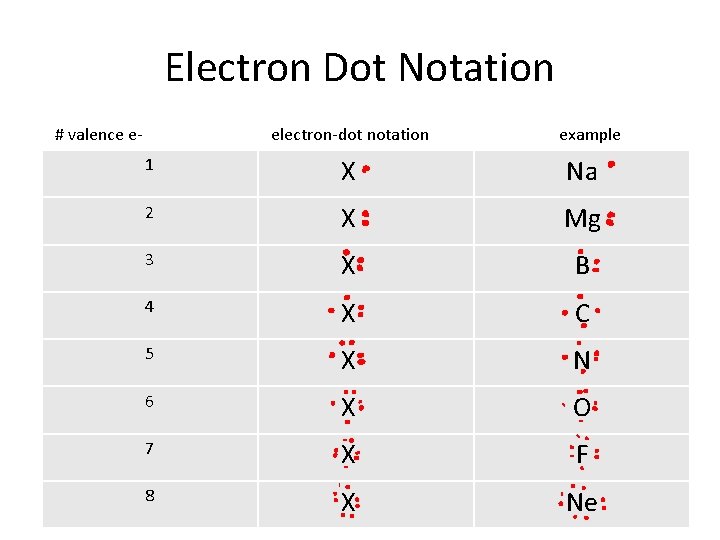
Electron Dot Notation # valence e- electron-dot notation example 1 X Na 2 X Mg 3 X B 4 X C 5 X N 6 X O 7 X F 8 X Ne
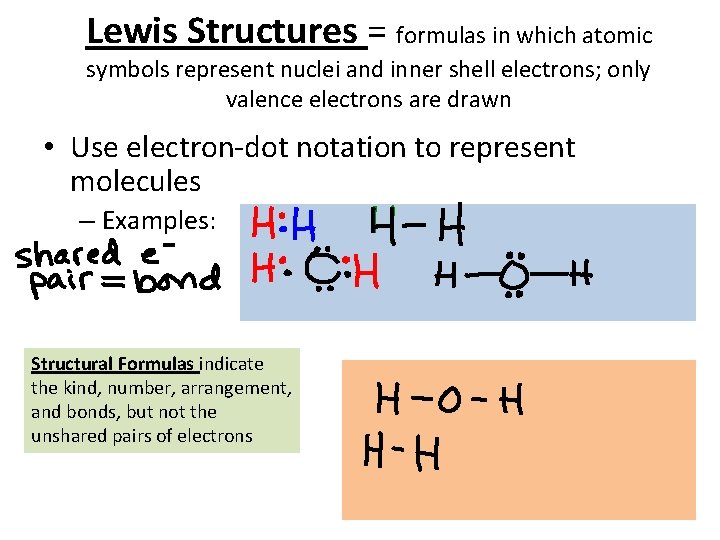
Lewis Structures = formulas in which atomic symbols represent nuclei and inner shell electrons; only valence electrons are drawn • Use electron-dot notation to represent molecules – Examples: Structural Formulas indicate the kind, number, arrangement, and bonds, but not the unshared pairs of electrons
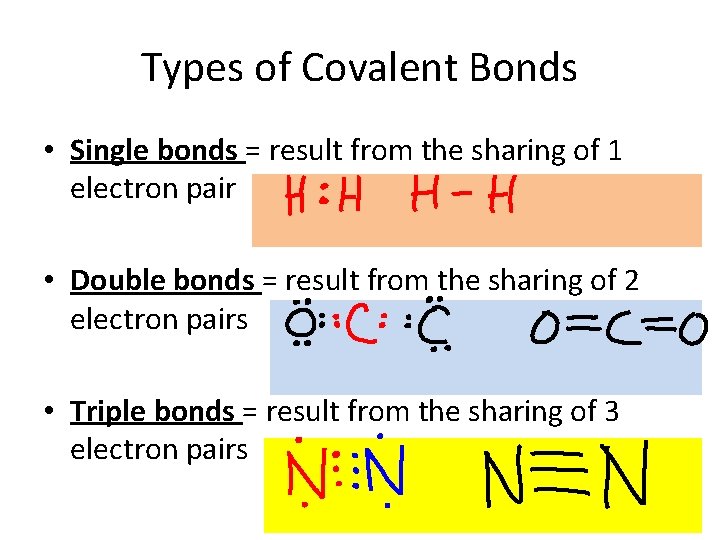
Types of Covalent Bonds • Single bonds = result from the sharing of 1 electron pair • Double bonds = result from the sharing of 2 electron pairs • Triple bonds = result from the sharing of 3 electron pairs
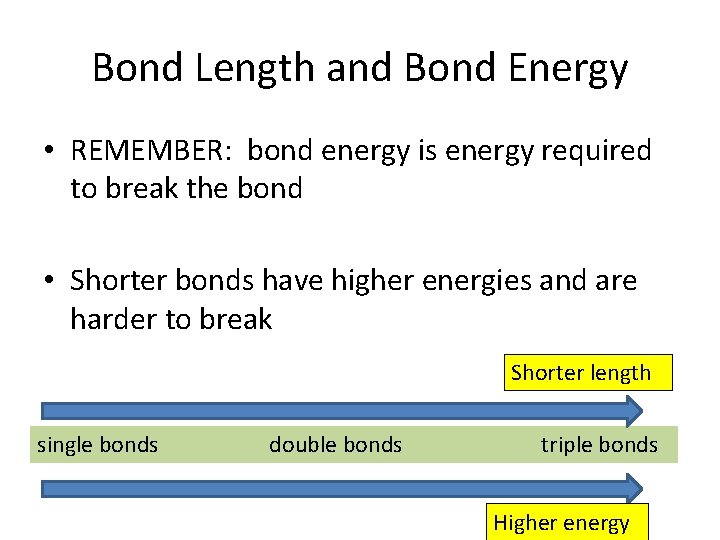
Bond Length and Bond Energy • REMEMBER: bond energy is energy required to break the bond • Shorter bonds have higher energies and are harder to break Shorter length single bonds double bonds triple bonds Higher energy
Molecular Geometry = the 3 -dimensional arrangement of a molecule’s atoms in space Geometry and bond polarity will determine molecular polarity and intermolecular forces. Forces of attraction between molecules
Intramolecular Forces • Forces between atoms or ions that keep compounds together – Ionic attraction – Sharing electrons
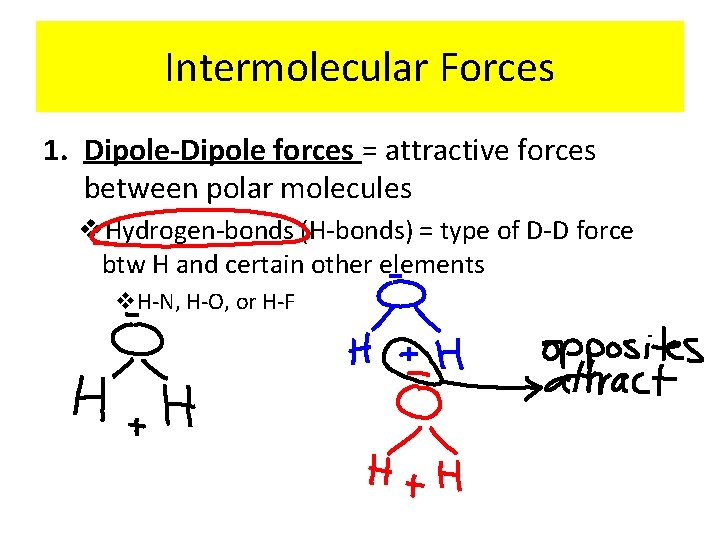
Intermolecular Forces 1. Dipole-Dipole forces = attractive forces between polar molecules v. Hydrogen-bonds (H-bonds) = type of D-D force btw H and certain other elements v. H-N, H-O, or H-F
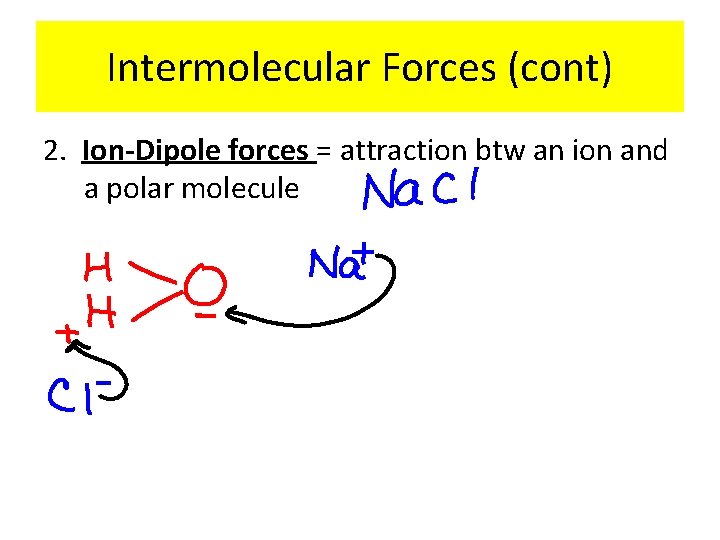
Intermolecular Forces (cont) 2. Ion-Dipole forces = attraction btw an ion and a polar molecule
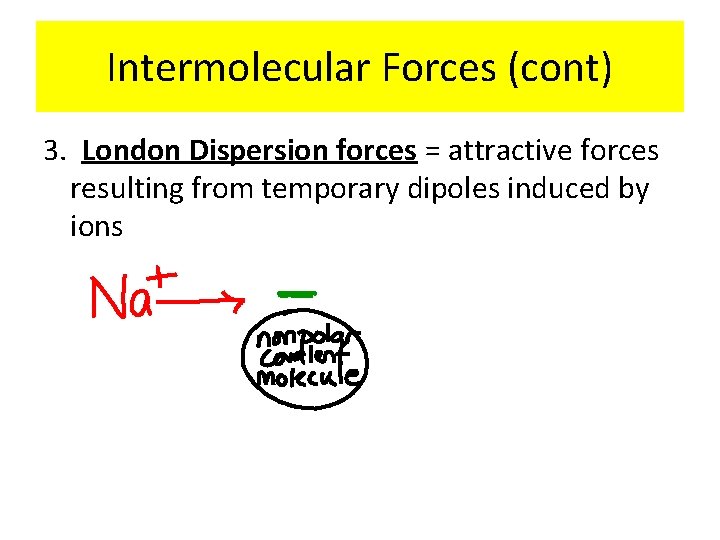
Intermolecular Forces (cont) 3. London Dispersion forces = attractive forces resulting from temporary dipoles induced by ions
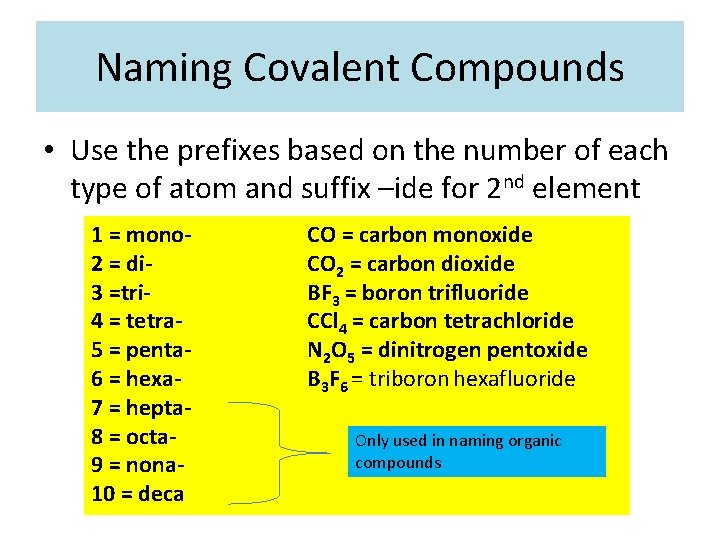
Naming Covalent Compounds • Use the prefixes based on the number of each type of atom and suffix –ide for 2 nd element 1 = mono 2 = di 3 =tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca CO = carbon monoxide CO 2 = carbon dioxide BF 3 = boron trifluoride CCl 4 = carbon tetrachloride N 2 O 5 = dinitrogen pentoxide B 3 F 6 = triboron hexafluoride Only used in naming organic compounds
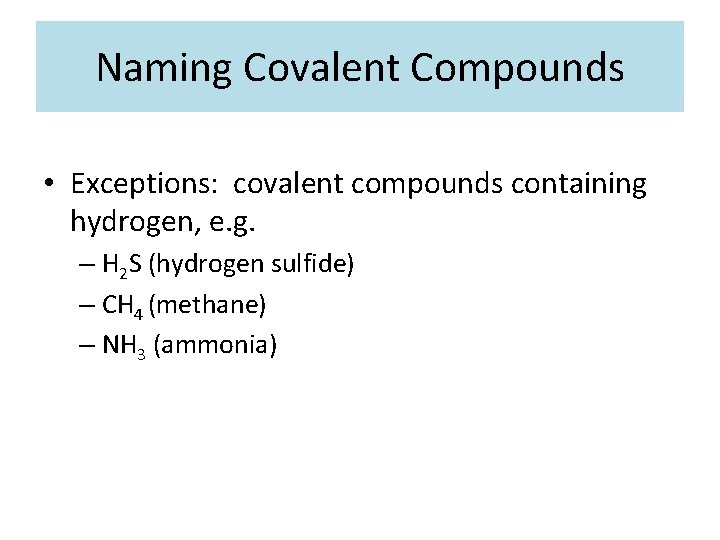
Naming Covalent Compounds • Exceptions: covalent compounds containing hydrogen, e. g. – H 2 S (hydrogen sulfide) – CH 4 (methane) – NH 3 (ammonia)
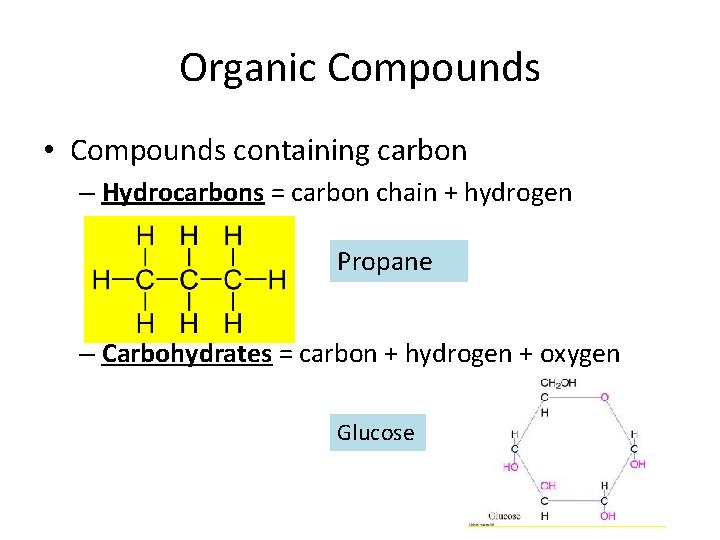
Organic Compounds • Compounds containing carbon – Hydrocarbons = carbon chain + hydrogen Propane – Carbohydrates = carbon + hydrogen + oxygen Glucose
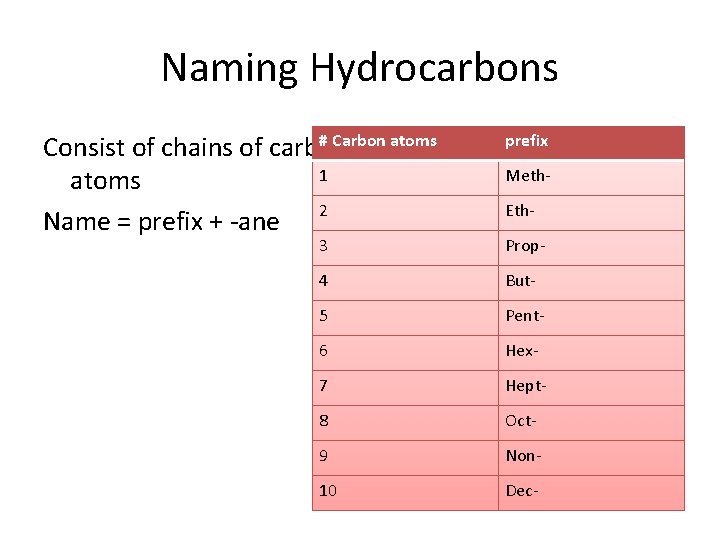
Naming Hydrocarbons # Carbon atoms Consist of chains of carbon 1 atoms Name = prefix + -ane 2 prefix Meth. Eth- 3 Prop- 4 But- 5 Pent- 6 Hex- 7 Hept- 8 Oct- 9 Non- 10 Dec-

Drawing Hydrocarbons
Ionic Compounds = Compounds consisting of a positive ion and a negative ion held together by the attraction of opposite electrical charges The overall charge of an ionic compound = 0 The + and - ions cancel each other
Formula Units • Molecules are the basic unit of covalent compounds (a molecule of water, H 2 O) • The smallest unit of an ionic compound is the formula unit (a formula unit of sodium chloride, Na. Cl)
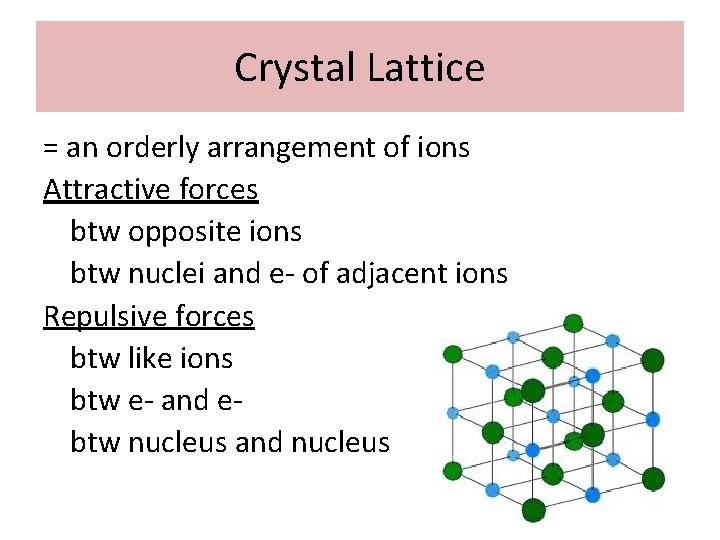
Crystal Lattice = an orderly arrangement of ions Attractive forces btw opposite ions btw nuclei and e- of adjacent ions Repulsive forces btw like ions btw e- and ebtw nucleus and nucleus
Lattice Energy and Bond Strength To compare bond strength in ionic compounds, we compare lattice energy Related to the energy in the bond = how tightly the ions in a crystal are held together Remember: higher energy bonds are harder to break
Molecular (covalent) vs. Ionic • Covalent and ionic bonds are strong attractions that hold the atoms in a compound together Molecules in Covalent Compounds Held together by intermolecular forces STRONGER Formula Units in Ionic Compounds Held together by attractive forces in a crystalline lattice
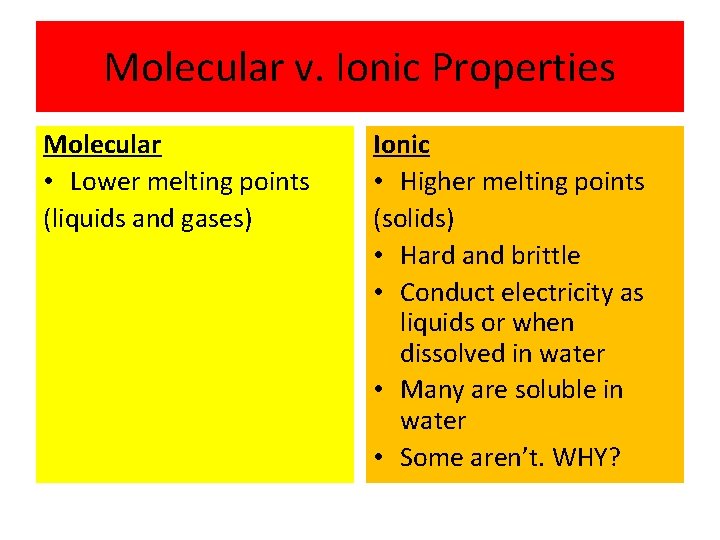
Molecular v. Ionic Properties Molecular • Lower melting points (liquids and gases) Ionic • Higher melting points (solids) • Hard and brittle • Conduct electricity as liquids or when dissolved in water • Many are soluble in water • Some aren’t. WHY?
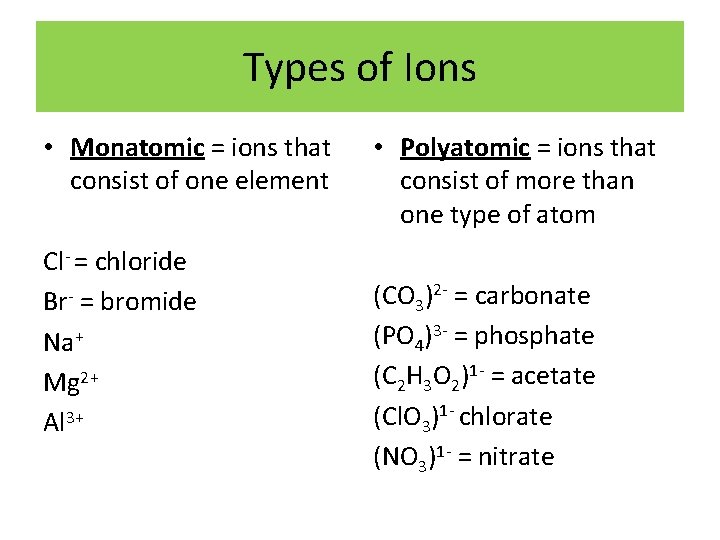
Types of Ions • Monatomic = ions that consist of one element Cl- = chloride Br- = bromide Na+ Mg 2+ Al 3+ • Polyatomic = ions that consist of more than one type of atom (CO 3)2 - = carbonate (PO 4)3 - = phosphate (C 2 H 3 O 2)1 - = acetate (Cl. O 3)1 - chlorate (NO 3)1 - = nitrate
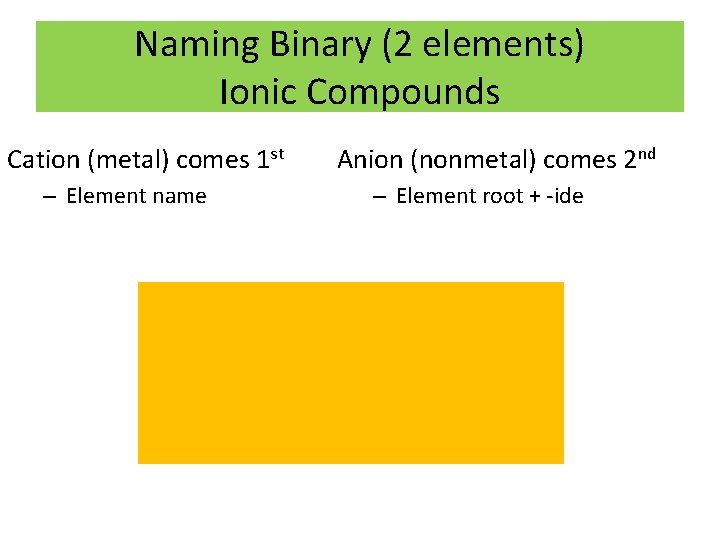
Naming Binary (2 elements) Ionic Compounds Cation (metal) comes 1 st – Element name Anion (nonmetal) comes 2 nd – Element root + -ide
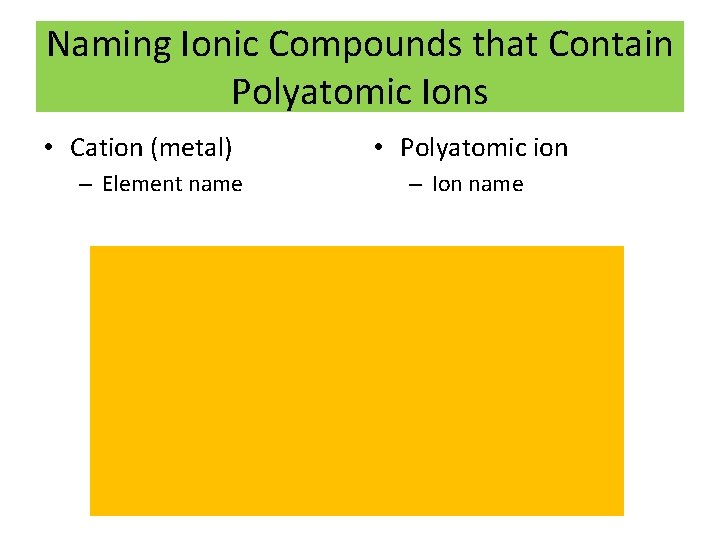
Naming Ionic Compounds that Contain Polyatomic Ions • Cation (metal) – Element name • Polyatomic ion – Ion name
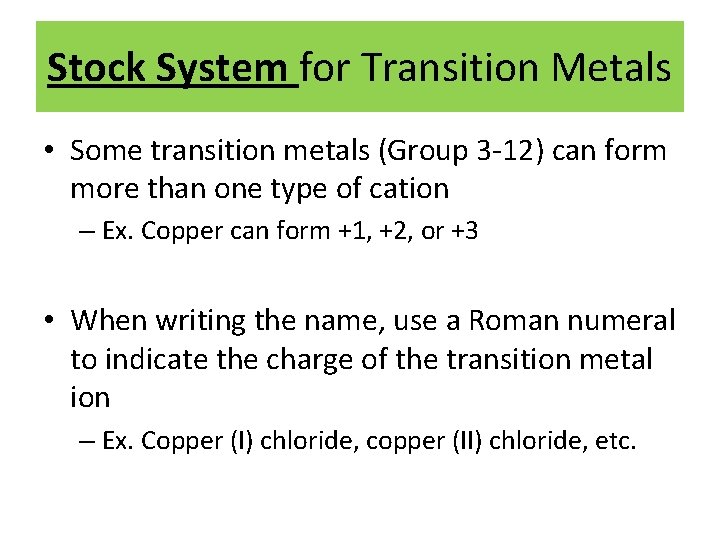
Stock System for Transition Metals • Some transition metals (Group 3 -12) can form more than one type of cation – Ex. Copper can form +1, +2, or +3 • When writing the name, use a Roman numeral to indicate the charge of the transition metal ion – Ex. Copper (I) chloride, copper (II) chloride, etc.
Writing Formulas for Ionic Compounds 1. Symbol for cation + symbol/formula for cation 2. Write the charges as super scripts 3. Cross the numbers down to subscripts
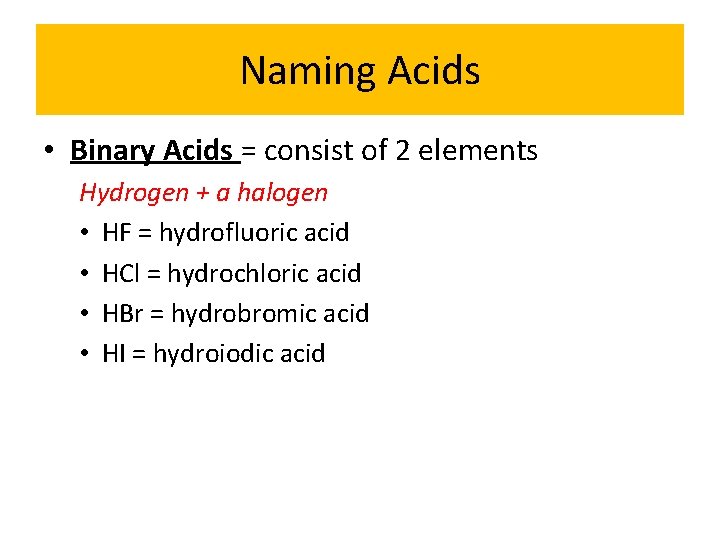
Naming Acids • Binary Acids = consist of 2 elements Hydrogen + a halogen • HF = hydrofluoric acid • HCl = hydrochloric acid • HBr = hydrobromic acid • HI = hydroiodic acid
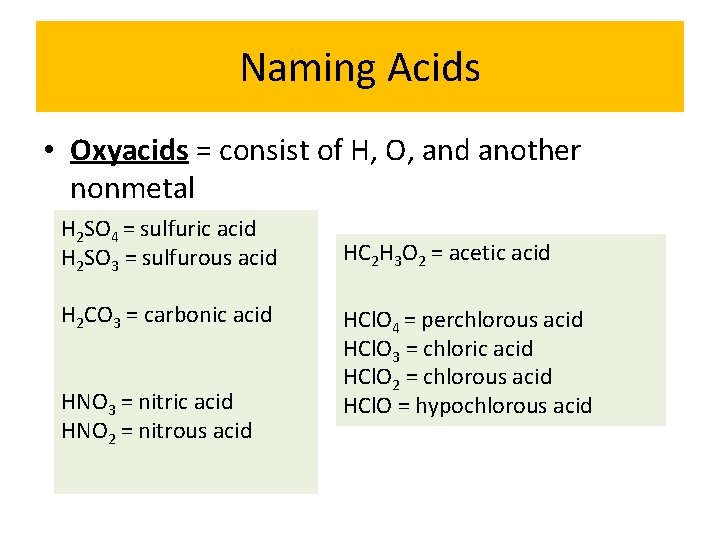
Naming Acids • Oxyacids = consist of H, O, and another nonmetal H 2 SO 4 = sulfuric acid H 2 SO 3 = sulfurous acid H 2 CO 3 = carbonic acid HNO 3 = nitric acid HNO 2 = nitrous acid HC 2 H 3 O 2 = acetic acid HCl. O 4 = perchlorous acid HCl. O 3 = chloric acid HCl. O 2 = chlorous acid HCl. O = hypochlorous acid
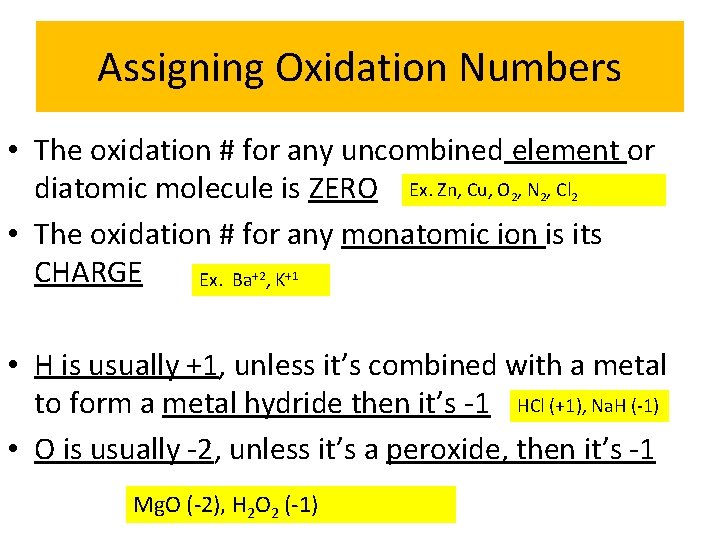
Assigning Oxidation Numbers • The oxidation # for any uncombined element or diatomic molecule is ZERO Ex. Zn, Cu, O 2, N 2, Cl 2 • The oxidation # for any monatomic ion is its CHARGE Ex. Ba+2, K+1 • H is usually +1, unless it’s combined with a metal to form a metal hydride then it’s -1 HCl (+1), Na. H (-1) • O is usually -2, unless it’s a peroxide, then it’s -1 Mg. O (-2), H 2 O 2 (-1)

Assigning Oxidation Numbers • In binary covalent compounds (nonmetal + nonmetal) the positive one is first and the negative one is second • The sum of the oxidation numbers for all atoms in a neutral compound is ZERO • The sum of the oxidation numbers in a polyatomic ion is equal to the CHARGE of the polyatomic ion

Practice • H 2 • Ca. Cl 2 • KCl. O 4
- Slides: 45