Chemical Bonds Drawing Ionic and Covalent Bonds Ionic

Chemical Bonds Drawing Ionic and Covalent Bonds

Ionic Bonds (Metal & Nonmetal) Draw the Bond Between Potassium and Oxygen EXAMPLE Potassium & Oxygen
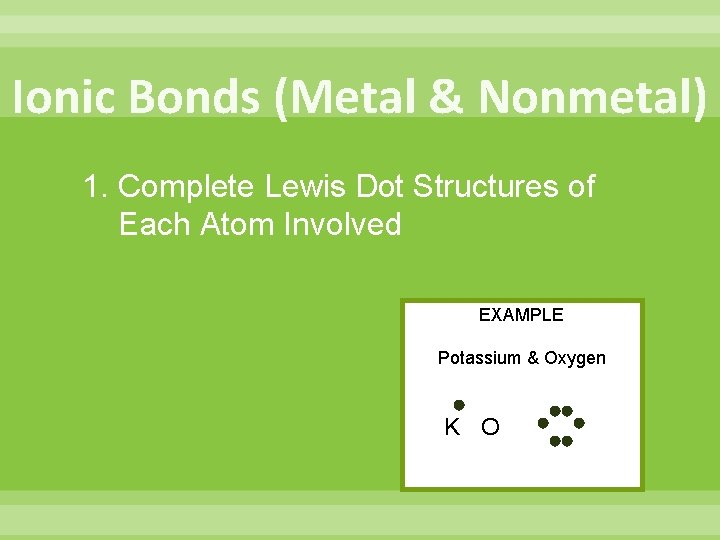
Ionic Bonds (Metal & Nonmetal) 1. Complete Lewis Dot Structures of Each Atom Involved EXAMPLE Potassium & Oxygen K O
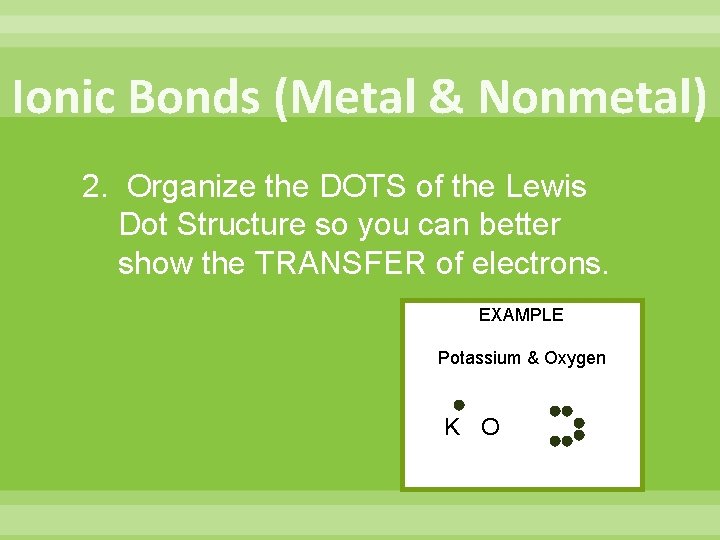
Ionic Bonds (Metal & Nonmetal) 2. Organize the DOTS of the Lewis Dot Structure so you can better show the TRANSFER of electrons. EXAMPLE Potassium & Oxygen K O
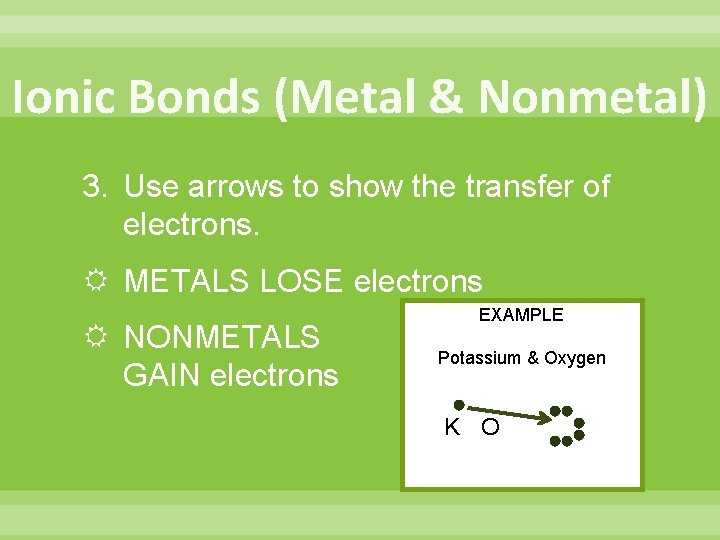
Ionic Bonds (Metal & Nonmetal) 3. Use arrows to show the transfer of electrons. METALS LOSE electrons NONMETALS GAIN electrons EXAMPLE Potassium & Oxygen K O
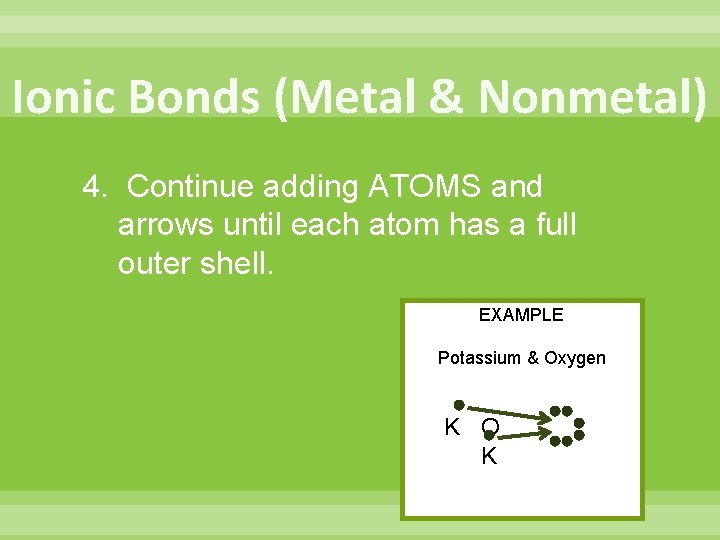
Ionic Bonds (Metal & Nonmetal) 4. Continue adding ATOMS and arrows until each atom has a full outer shell. EXAMPLE Potassium & Oxygen K O K
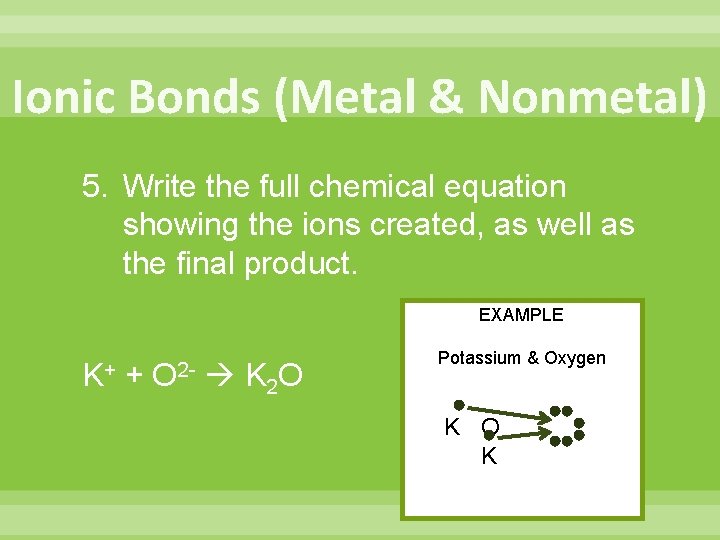
Ionic Bonds (Metal & Nonmetal) 5. Write the full chemical equation showing the ions created, as well as the final product. EXAMPLE K+ + O 2 - K 2 O Potassium & Oxygen K O K
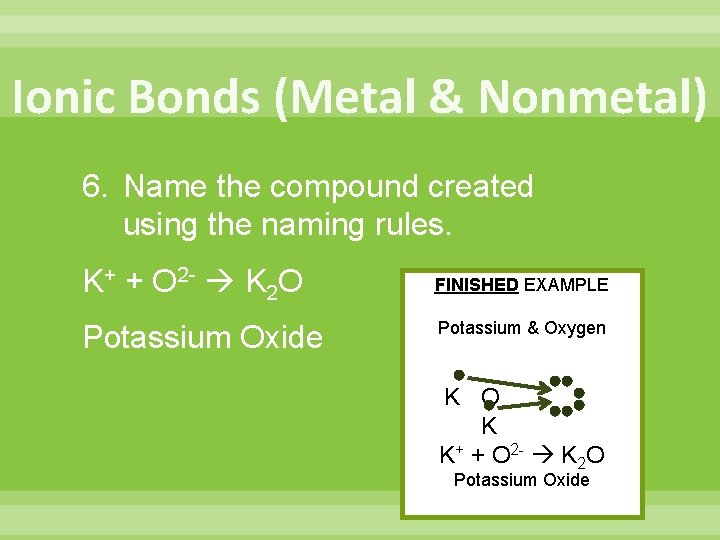
Ionic Bonds (Metal & Nonmetal) 6. Name the compound created using the naming rules. K+ + O 2 - K 2 O FINISHED EXAMPLE Potassium Oxide Potassium & Oxygen K O K K+ + O 2 - K 2 O Potassium Oxide
Covalent Bonds (2 Nonmetals) Draw the Bond for H 2 S EXAMPLE H 2 S
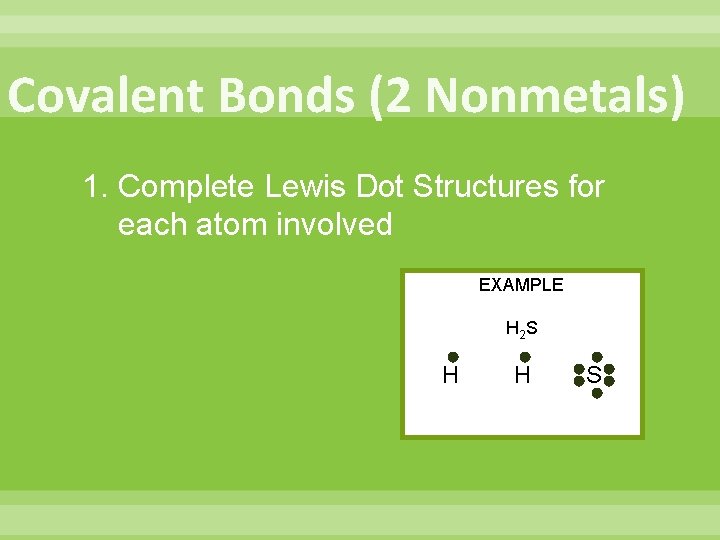
Covalent Bonds (2 Nonmetals) 1. Complete Lewis Dot Structures for each atom involved EXAMPLE H 2 S H H S
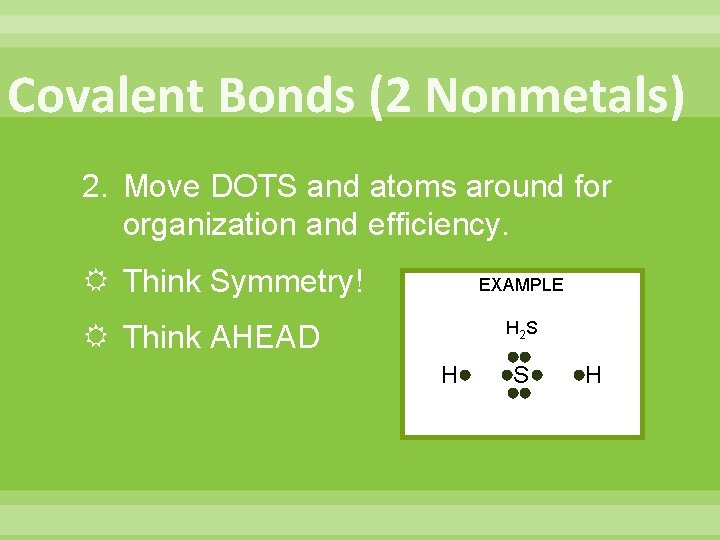
Covalent Bonds (2 Nonmetals) 2. Move DOTS and atoms around for organization and efficiency. Think Symmetry! EXAMPLE H 2 S Think AHEAD H S H
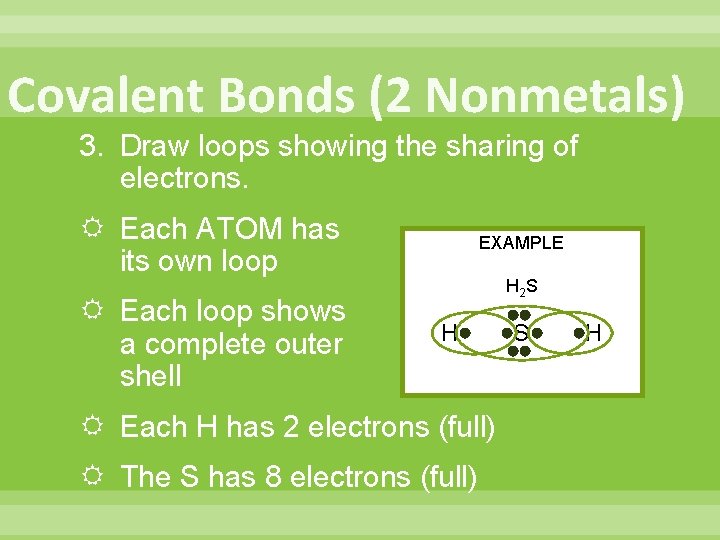
Covalent Bonds (2 Nonmetals) 3. Draw loops showing the sharing of electrons. Each ATOM has its own loop Each loop shows a complete outer shell EXAMPLE H 2 S H Each H has 2 electrons (full) The S has 8 electrons (full) S H
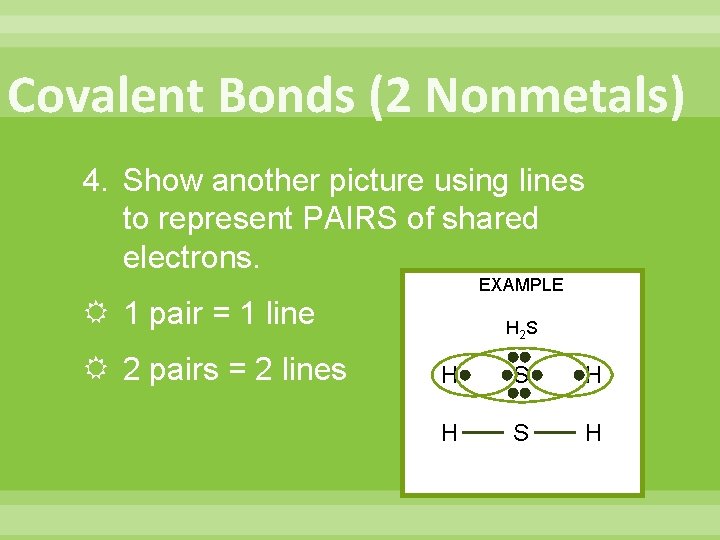
Covalent Bonds (2 Nonmetals) 4. Show another picture using lines to represent PAIRS of shared electrons. EXAMPLE 1 pair = 1 line 2 pairs = 2 lines H 2 S H H S H
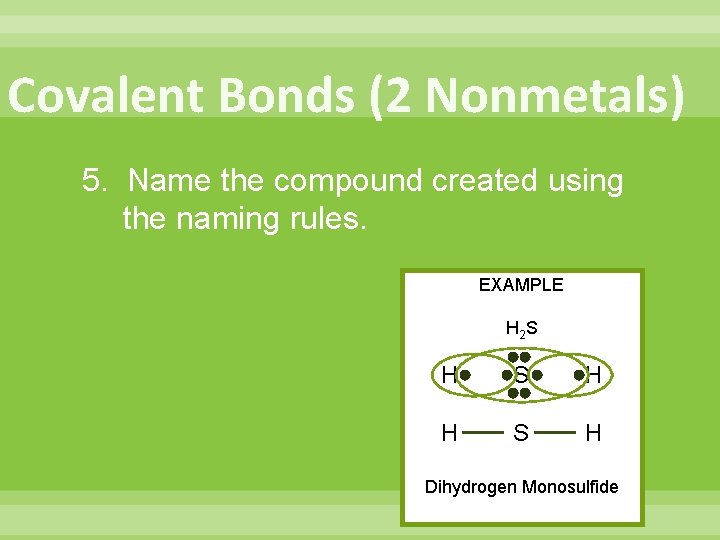
Covalent Bonds (2 Nonmetals) 5. Name the compound created using the naming rules. EXAMPLE H 2 S H H S H Dihydrogen Monosulfide
- Slides: 14