Chemical Bonds Covalent Polar Covalent Chemical Bonds Chemical
Chemical Bonds (Covalent) (Polar Covalent)
Chemical Bonds Chemical bonds are formed between atoms by the interaction of their respective electrons. There are three types of intramolecular bonds (bonds that molecule in a that hold aatoms together): molecule Ionic Bonds Covalent Bonds Polar Covalent Bonds
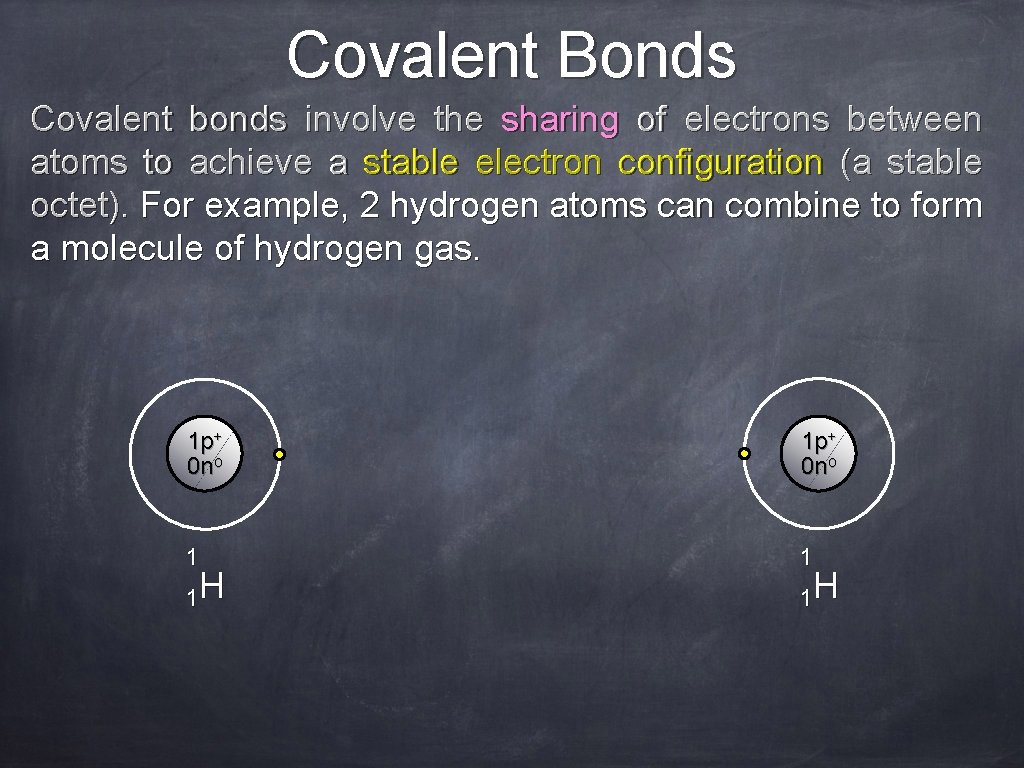
Covalent Bonds Covalent bonds involve the sharing of electrons between atoms to achieve a stable electron configuration (a stable octet). For example, 2 hydrogen atoms can combine to form a molecule of hydrogen gas. 1 p+ 0 no 1 1 1 H 1 H

Covalent Bonds Covalent bonds involve the sharing of electrons between atoms to achieve a stable electron configuration (a stable octet). For example, 2 hydrogen atoms can combine to form a molecule of hydrogen gas. 1 p+ 0 no H 2

Covalent Bonds Each hydrogen atom has one electron of its own and shares, , for some time shares time, , the electron of the other hydrogen atom. That is, for some time each hydrogen atom has a full outer energy level. Let’s review that. H 1 p+ 0 no A single bond is formed H 2 shared) (two electrons (Nelson Biology 12 animation) In this example, there is equal sharing of electrons, so this bond is said to be purely covalent.
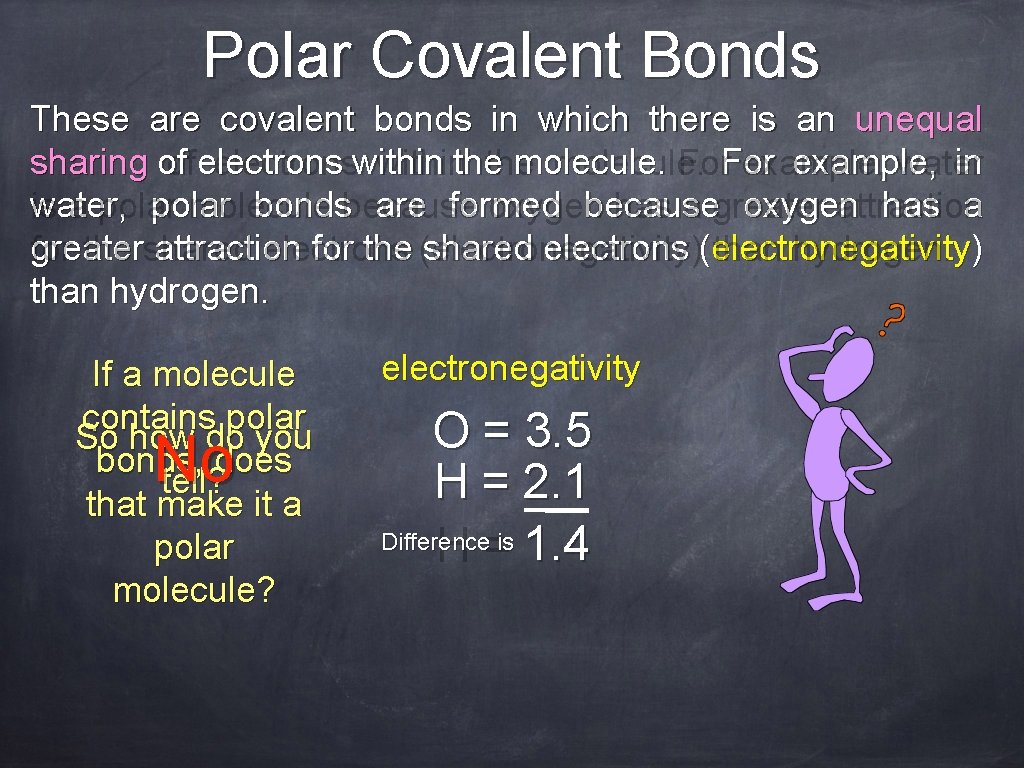
Polar Covalent Bonds These are covalent bonds in which there is an unequal sharing of of electronswithinthe molecule. For example, water in water, is a polar molecule bondsbecause are formed oxygen because has a greater oxygen attraction has a greater for the shared attraction electrons for the (electronegativity) shared electrons (electronegativity than hydrogen. ) than hydrogen. If a molecule contains polar So how do you bonds, does tell? that make it a polar molecule? No electronegativity O = 3. 5 H = 2. 1 Difference H =is 1. 4

Polar Covalent Bonds These are covalent bonds in which there is an unequal sharing of of electronswithinthe molecule. For example, water in water, is a polar molecule bondsbecause are formed oxygen because has a greater oxygen attraction has a greater for the shared attraction electrons for the (electronegativity) shared electrons (electronegativity than hydrogen. ) than hydrogen. Step 1: Draw a Lewis Dot Diagram for each atom. Step 2: Determine the structural formula of the molecule. H H O

Polar Covalent Bonds These are covalent bonds in which there is an unequal sharing of of electronswithinthe molecule. For example, water in water, is a polar molecule bondsbecause are formed oxygen because has a greater oxygen attraction has a greater for the shared attraction electrons for the (electronegativity) shared electrons (electronegativity than hydrogen. ) than hydrogen. Step 1: Draw a Lewis Dot Diagram for each atom. Step 2: Determine the structural formula of the molecule. 2 H H H O OO H HH That H doesn’t H work. 2 3 That works!
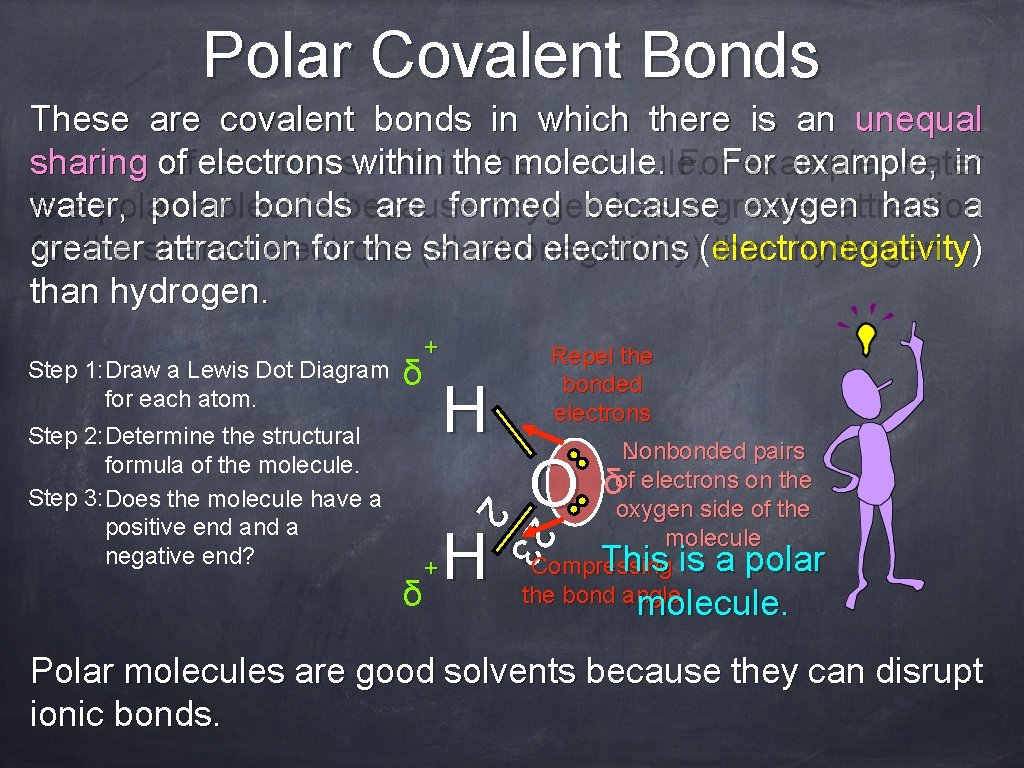
Polar Covalent Bonds These are covalent bonds in which there is an unequal sharing of of electronswithinthe molecule. For example, water in water, is a polar molecule bondsbecause are formed oxygen because has a greater oxygen attraction has a greater for the shared attraction electrons for the (electronegativity) shared electrons (electronegativity than hydrogen. ) than hydrogen. Step 1: Draw a Lewis Dot Diagram for each atom. δ H Repel the bonded electrons Nonbonded pairs δof electrons on the oxygen side of the molecule This is a polar Compressing the bond angle molecule. 2 Step 2: Determine the structural formula of the molecule. Step 3: Does the molecule have a positive end a negative end? + H 2 3 δ + O molecule. Polar molecules are good solvents because they can disrupt ionic bonds.
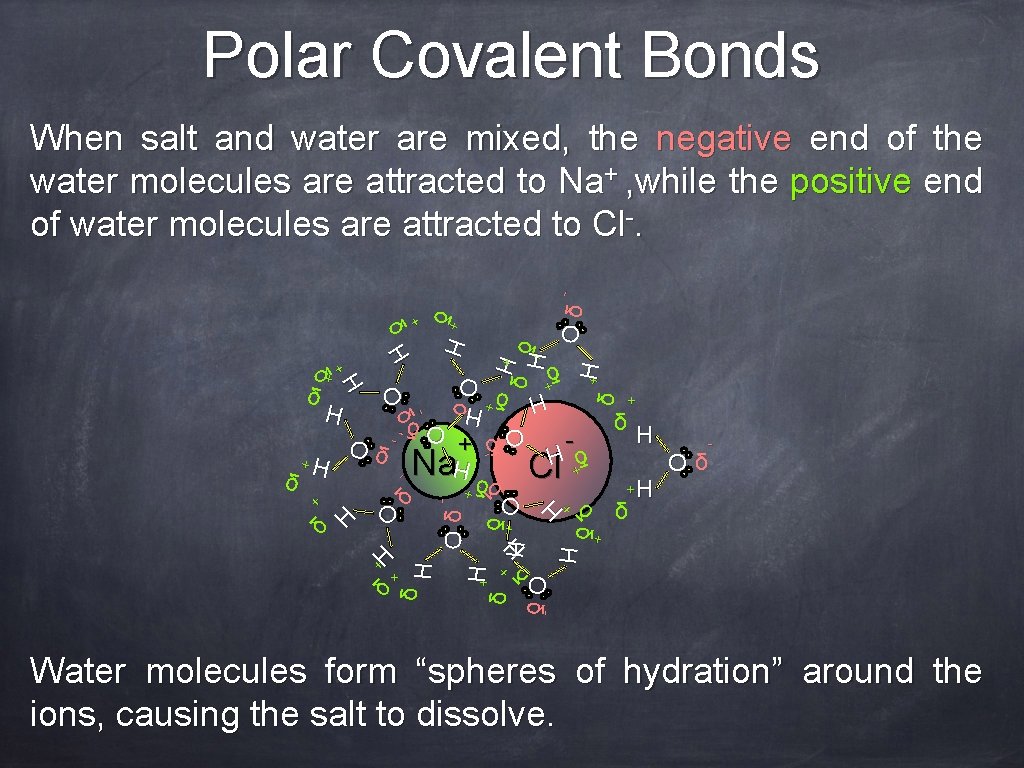
Polar Covalent Bonds δ+ + H δ H O δOδ H H δ+ O H+ δ δ - H +H Oδ - δ + δ H - - O δ- +H δ + δ δ Oδ - - + H H δ +H + δ H Cl + O δ Na δ +H H + δ + δ δ H + Oδ - δ+ H O δ- - δ +H O δ H + H δ + δ H H + δ δ + Oδ - When salt and water are mixed, the negative end of the water molecules are attracted to Na+ , while the positive end of water molecules are attracted to Cl-. Water molecules form “spheres of hydration” around the ions, causing the salt to dissolve.
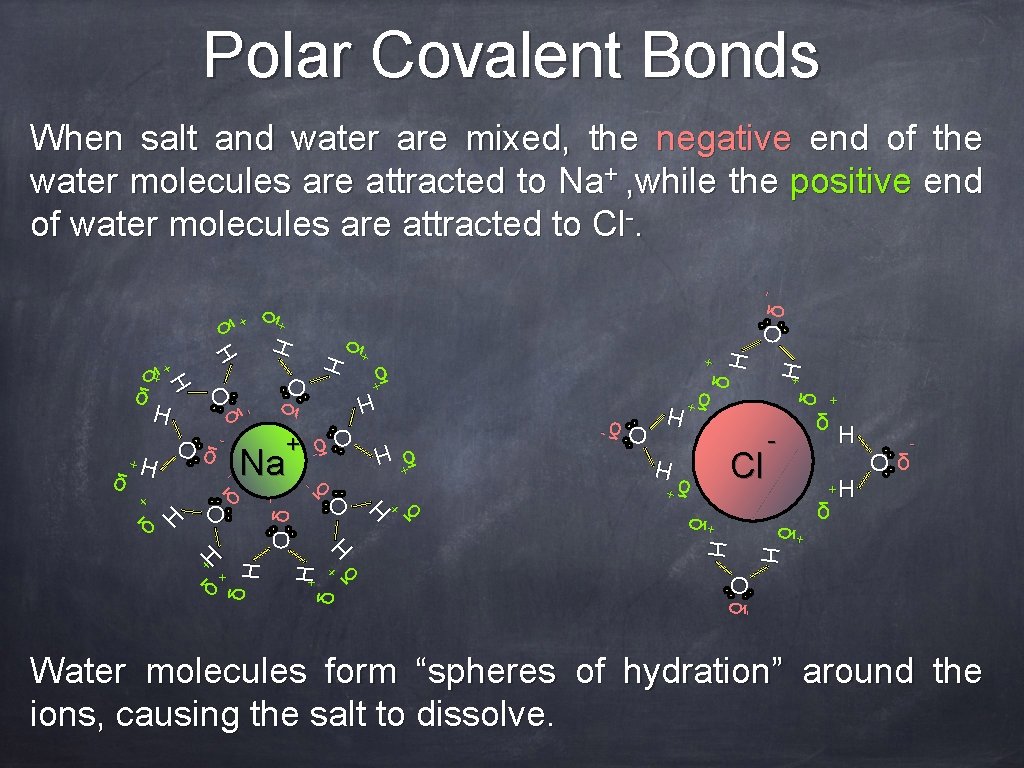
Polar Covalent Bonds - H+ δ O +H H + δ δ + - H δ +H Oδ - + H δ H - - O δ- +H δ + δ δ H Oδ δ+ δ H Oδ - H O δ- - H H δ + δ O Cl + δ +H + δ H δ+ + + Na δ + δ δ H + Oδ - δ+ H O δ- δ +H - H O δ + H δ + δ H H + δ δ + Oδ - When salt and water are mixed, the negative end of the water molecules are attracted to Na+ , while the positive end of water molecules are attracted to Cl-. Water molecules form “spheres of hydration” around the ions, causing the salt to dissolve.

Polar Covalent Bonds When salt and water are mixed, the negative end of the water molecules are attracted to Na+ , while the positive end of water molecules are attracted to Cl-. Let’s see that again (Nelson Biology 12 animation) Water molecules form “spheres of hydration” around the ions, causing the salt to dissolve.

Ionic, Covalent or Polar Covalent? The type of bond that forms is determined by the difference in the electronegativities of the two atoms involved. If there is essentially no difference (< 0. 5), the bond is considered covalent. H H 2. 1 - 2. 1 = 0 O H 3. 5 - 2. 1 = 1. 4 If the difference is between 0. 5 and 1. 7, the bond is considered polar covalent. If the difference is equal to or greater than 1. 7, the bond is considered ionic. + Na Cl - 2. 9 - 1. 0 = 1. 9
Grade 12 Biology - SB 14 U 1 University Preparation Mr. Manbodh

- Slides: 15