Chemical Bonds and Balancing Equations EQ Why do

Chemical Bonds and Balancing Equations EQ: Why do chemicals react? What is the difference between compounds, mixtures, and solutions?

Important Terms l l Molecule- is two or more atoms combined and are physically attached. Compound- is when two or more elements are combined by chemical bonds. l Usually found as a solid. l Ex. l H 2 O = Water l Ex. Na. Cl = Sodium Chloride (Table Salt) Solution- is when two or more substances are combined by dissolving a substance in pure water. l Usually found as a liquid. l Can be separated by boiling or distillation. l Ex. Salt Water; Sugar Water

Important Terms l l l Mixture- is when two or more substances are combined but ARE NOT chemically bonded. l Can be both solids and liquids. Heterogeneous Mixture- Not an even mix throughout. l Ex. Fruit Salad, Pepper, Lucky Charms Homogeneous Mixture - Even mix throughout. l Ex. Steel and Clear Salt Water

Recall Why do elements react? l To achieve a more stable electron configuration (full outer shell) l

What types of bonds are created? l Ionic - electrons are transferred from one element and give to another. l Metal joins with a Nonmetal STRONGEST CHEMICAL BOND l New terms l Cation-positive element l Anion-negative element l So sodium is a cation and chloride is the anion in Na. Cl(salt)

Covalent Bond Atoms share electrons: NO ELECTRONS ARE SHIFTED OR REMOVED l Can create either single or double bonds to achieve full outer shell l Most Commonly done with gases that do not have a full outer shell (O 2 Cl 2 N 2 ) l Atoms of the gases join up to achieve stability (weak bond, easily broken) l
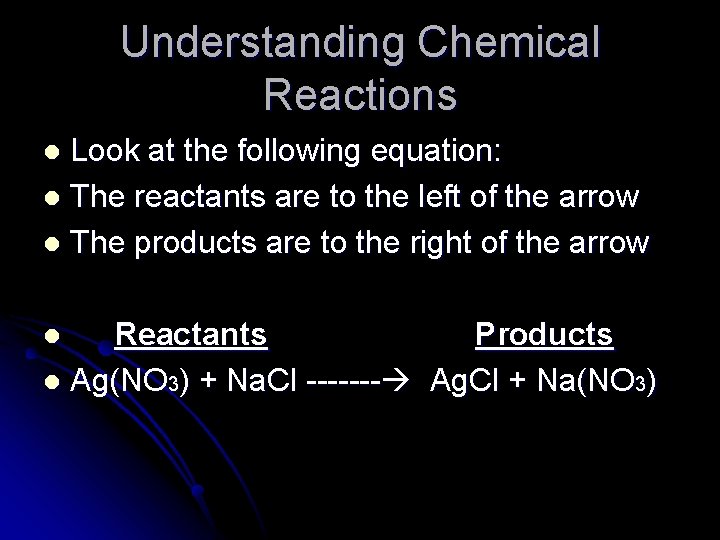
Understanding Chemical Reactions Look at the following equation: l The reactants are to the left of the arrow l The products are to the right of the arrow l Reactants Products l Ag(NO 3) + Na. Cl ------- Ag. Cl + Na(NO 3) l

Now list the number of atoms there are present for the Reactants and Products l Reactants l l l Ag N O Na Cl l Products l l l Ag N O Na Cl

Now write the number of atoms of each element l l l REACTANTS 1 Ag 1 N 3 O 1 Na 1 Cl PRODUCTS 1 Ag 1 N 3 O 1 Na 1 Cl

Counting Atoms Notice that ALL The atoms listed are EQUAL in number on BOTH SIDES of the reaction l In a chemical formula you assume the number 1 when no other number is listed l

Counting Atoms l Notice that oxygen has 3 as its number! This is because oxygen is part of a polyatomic molecule. There are 3 oxygen atoms joined with the formula l Diatomic Molecule – “two” atoms combined to make a molecule. l Polyatomic Molecule – “three or more” atoms combined to make a molecule.

Now consider this formula l l l CH 4 + O 2 --------> CO 2 + H 2 O Reactants C H O Products C H O

How many atoms are listed for the products and reactants Reactants l C– 1 l H– 4 l O– 2 Products l C– 1 l H– 2 l O– 3 THIS CAN NOT HAPPEN! You must Balance the equation so that numbers of atoms for the reactants equal the same number for the products.
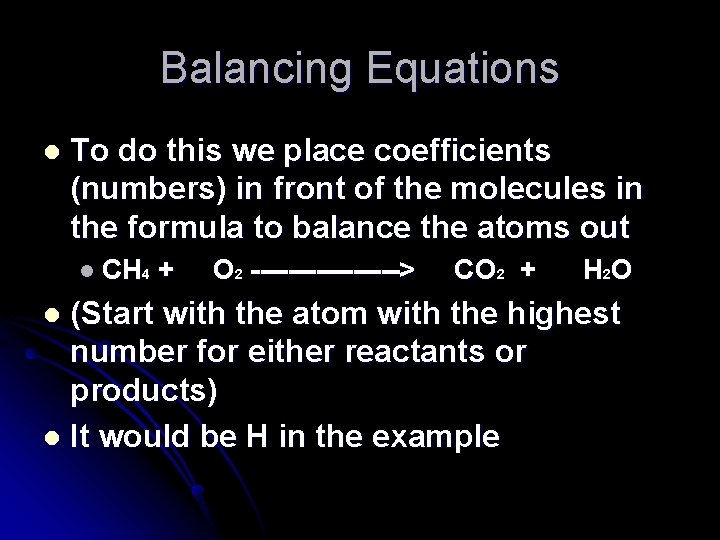
Balancing Equations l To do this we place coefficients (numbers) in front of the molecules in the formula to balance the atoms out l CH 4 + O 2 --------> CO 2 + H 2 O (Start with the atom with the highest number for either reactants or products) l It would be H in the example l

Balancing Equations l l l Reactants Products 1 C 1 C 4 H 2 H 2 O 3 O We need 2 more H in the products so we place a 2 in front of the water molecule CH 4 + O 2 --------> CO 2 + 2 H 2 O

Now Recheck l l l Reactants Products 1 C 4 H 2 O Now we need to balance out the Oxygen in the Reactants. CH 4 + 2 O 2 --------> CO 2 + 2 H 2 O 1 C 4 H 4 O

Recheck l Reactants 1 C 4 H l 4 O l l l It is now balanced!!! Products 1 C 4 H 4 O

Exit Slip l Balance this equation (its harder than it looks) l Al + O 2 ------> Al 2 O 3
- Slides: 18