Chemical Bonding What Why When Which Where How

Chemical Bonding What? Why? When? Which? Where? How? Mod. H U. 3 L 3&4

3 Types of Bonding • Ionic Bonding • Covalent Bonding • Metallic Bonding
Bonding is… • the transfer or sharing of electrons • concerned with the Valence Electrons

Ionic Bonding • Ionic bonding is an electrical attraction between two oppositely charged atoms.

When. . • Lose an electron - Atom is Positive • Positive Ion is called Cation ………………………………………………………………………………………………………………… • Gain an electron - Atom is Negative • Negative Ion is called Anion

Characteristics of Ionic Compounds • 1) Crystalline solid at room temp. • 2) Have higher melting and boiling points compared to covalent compounds. • 3)Conduct electrical current in solution state. • 4)Extremely polar (charged) bonds. • 5)Most are soluble in water.

Magic # • The magic number is 8 • Every atom wants to gain electrons up to 8, or lose electrons to equal zero, In order to be stable. • Exception= Hydrogen= only needs two electrons
Lewis Dot Structure • 1) Write the Atomic Symbol • 2)Count up the number of valence electrons. • 3)Draw a dot around the symbol for each valence electron.

Helpful Hint • An easy way to check if a bond is Ionic: • One atom must be from group 1, 2, or 13 on the periodic table and the other atom from groups 15 -17.

Oxidation Number (Charge) Group 1 2 13 14 15 16 17 18 Charge +1 +2 +3 -4 -3 -2 -1 0

Covalent Bonds • Covalent Bonds are formed as a result of the sharing of one or more pairs of valence electrons. • Each atom donates half of the electrons to be shared.

2 forms of covalent bonds • 1) Polar Covalent - unequal sharing of electrons • 2) Non-Polar Covalent – equal sharing of electrons between atoms.

Is held together by
Single Covalent Bond • A single covalent bond would be the sharing of two electrons between the 2 bonded atoms. • H-H H-Cl F-F • The dash line is symbolic of the bonding pair. • One line equals 2 electrons
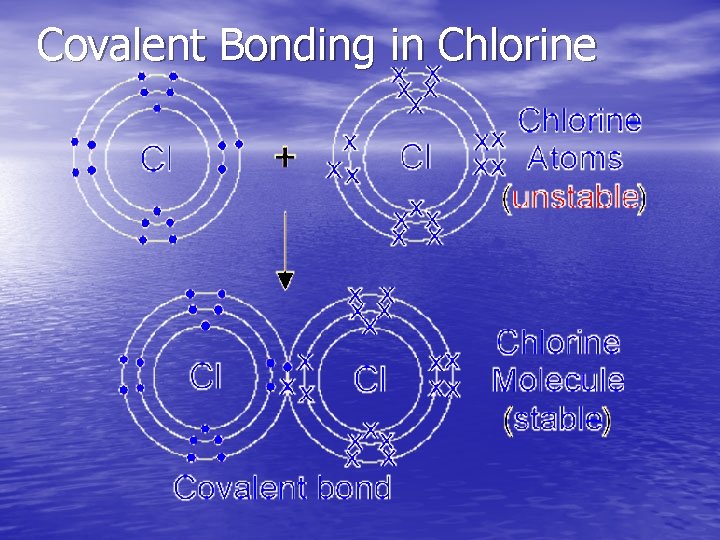
Covalent Bonding in Chlorine
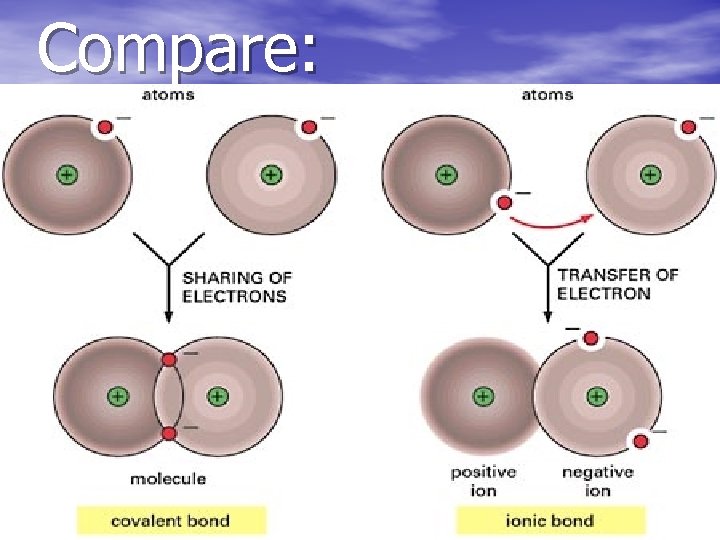
Compare:
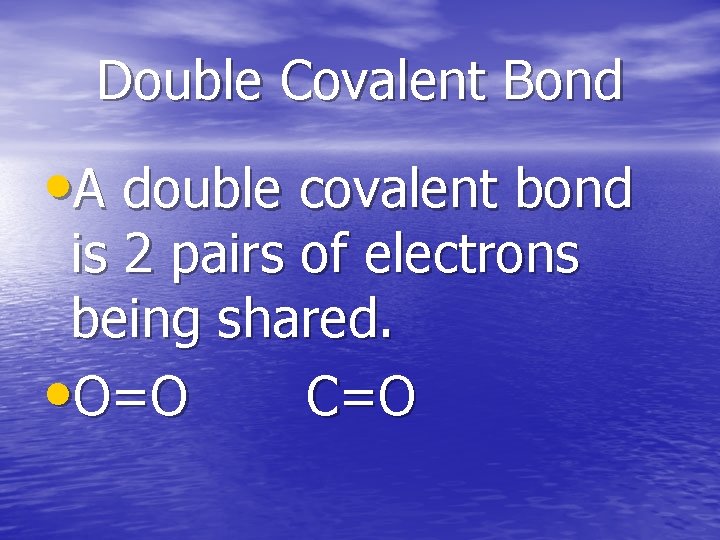
Double Covalent Bond • A double covalent bond is 2 pairs of electrons being shared. • O=O C=O
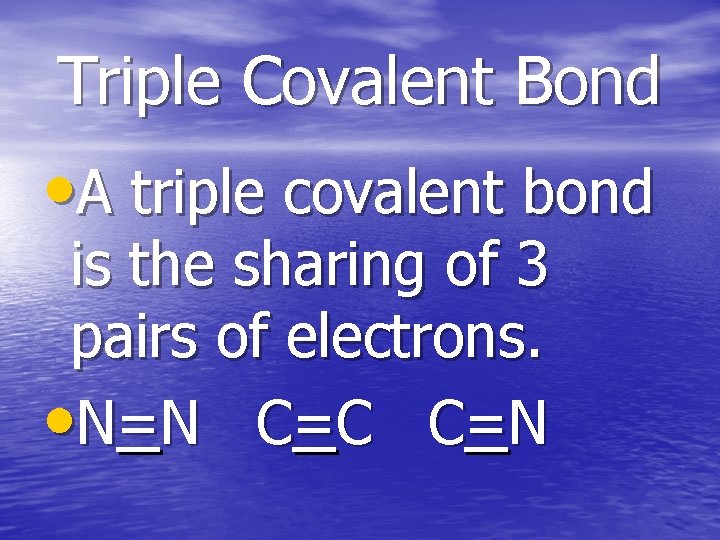
Triple Covalent Bond • A triple covalent bond is the sharing of 3 pairs of electrons. • N=N C =C C =N
Covalent bonds form molecules A molecule is a neutral group of atoms held together by covalent bonds Examples: water molecules, oxygen molecules…. .
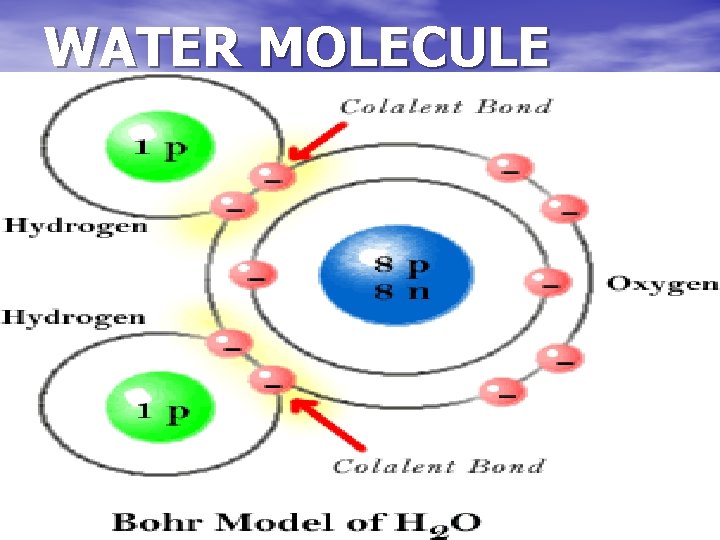
WATER MOLECULE
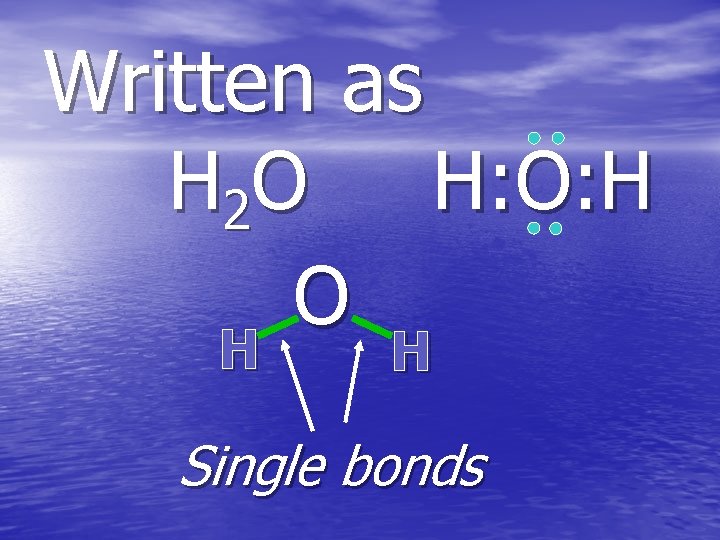
Written as H 2 O H: O: H O H H H Single bonds
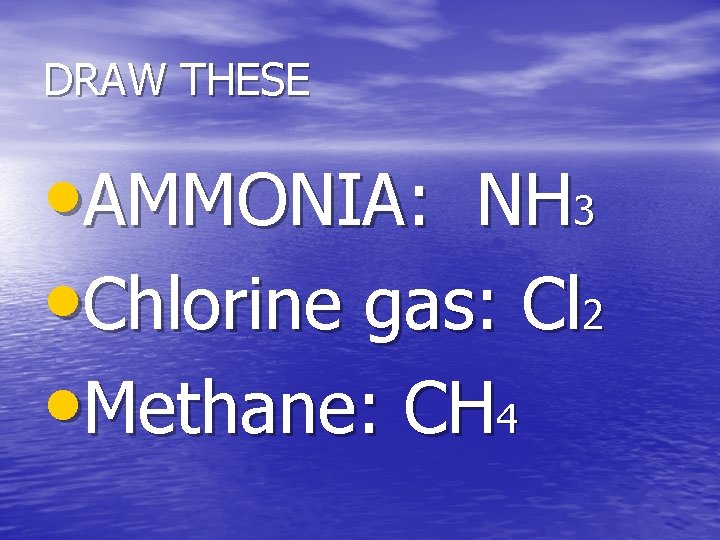
DRAW THESE • AMMONIA: NH 3 • Chlorine gas: Cl 2 • Methane: CH 4
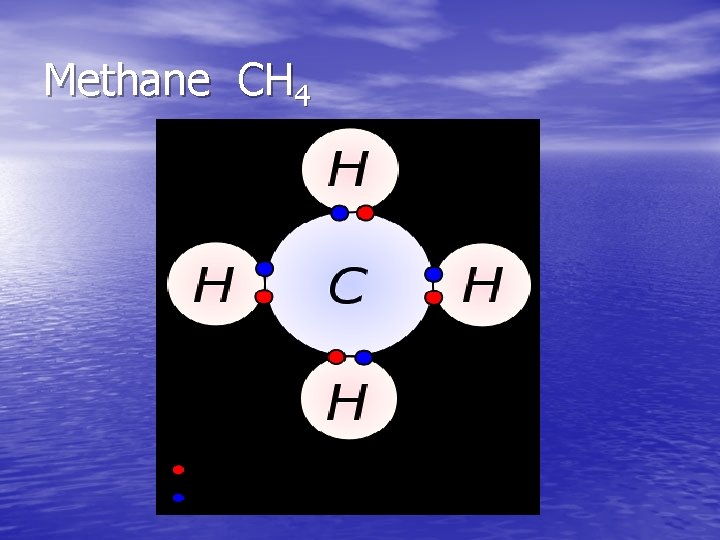
Methane CH 4
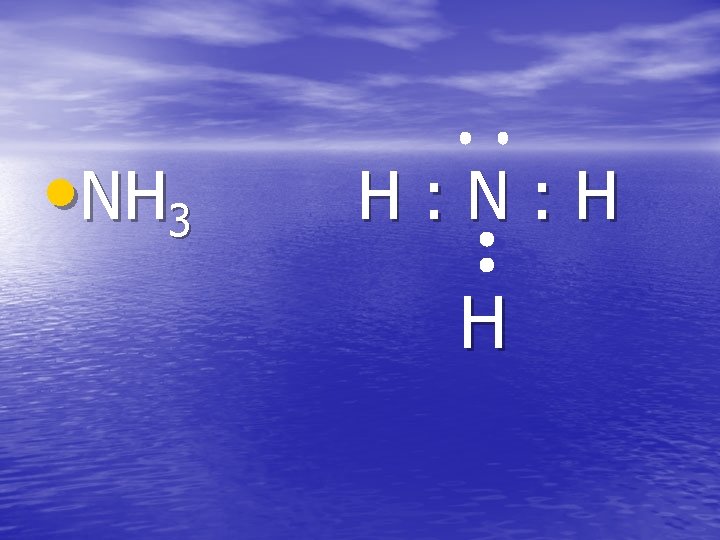
• NH 3 H: N: H H

Properties of covalent bonds: • Low melting point, boiling point • Brittle
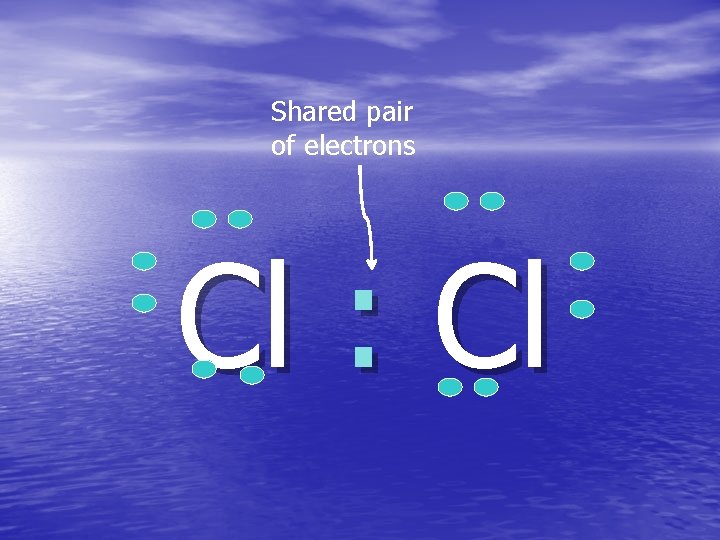
Shared pair of electrons Cl : Cl

Metallic Bond • The metallic bond occurs only between metal atoms. • In this type of bond the valence electrons overlap and are free to move about • The electron clouds of all metals participating in the bond. • It is similar to a “Sea of Electrons”.
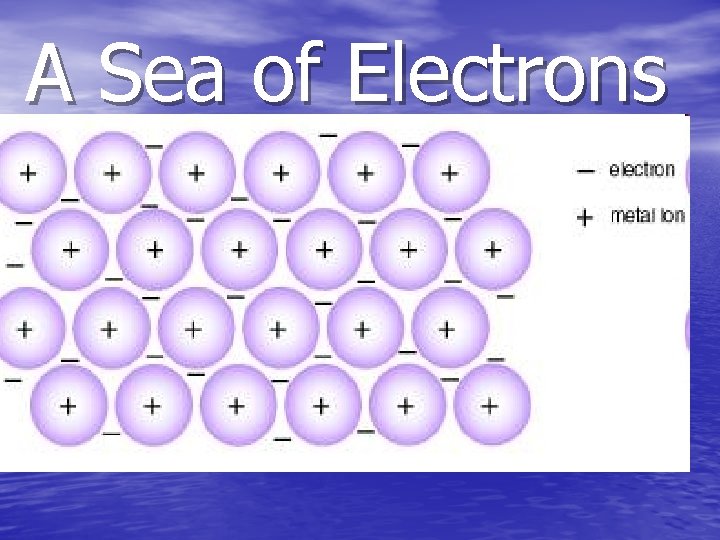
A Sea of Electrons
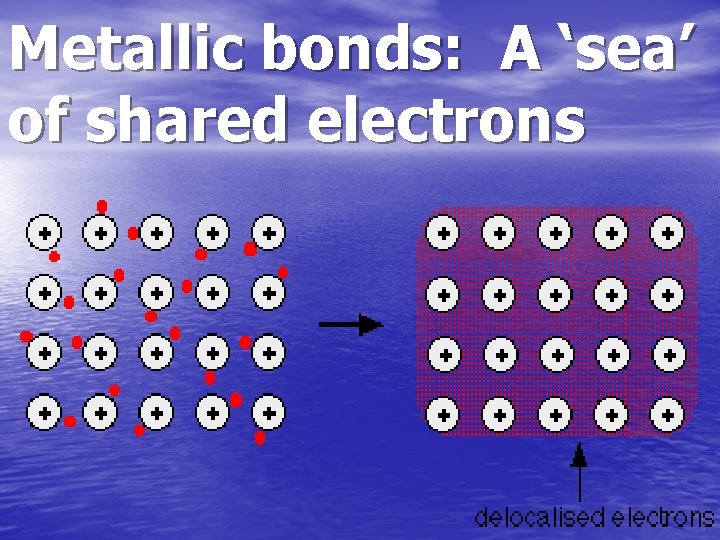
Metallic bonds: A ‘sea’ of shared electrons

Electrons can move freely • Metallic bonds account for the properties of metal Conductivity, ductility, malleability, higher temps • Example: paper clips!
- Slides: 30