CHEMICAL BONDING What forces hold atoms and molecules

CHEMICAL BONDING What forces hold atoms and molecules together?

What is a bond? • Bond: The force holding 2 or more atoms together making them function as a unit. • Examples: Ionic, covalent, metal • Bond Energy: The energy required to break a given chemical bond • A measure of the general strength of the bond
Three General Types of Bonds • Covalent bond: the sharing of valence electron pairs between atoms. Usually found between nonmetals. • Ionic bond: the transfer of valence electrons from a metal to a nonmetal. • Metallic bond: attractive force holding pure metals together.

Why do atoms bond? • Octet Rule: • Atoms tend to gain, lose, or share electrons until they are surrounded by 8 valence electrons (4 electron pairs). • All noble gases except He have an s 2 p 6 configuration. • Hydrogen follows the duet rule.
Ionic Bond Formation Non-Metal Neutral atoms come near each other. Electron(s) are transferred from the Metal atom to the Non-metal atom. They stick together because of electrostatic forces, like magnets.
Why does a metal & nonmetal form an ionic bond? • The difference in electronegativity determine bond type. • Electronegativity: the tendency of an atom in a molecule to attract shared electrons to itself. • High Electronegativity – high attraction of electrons • Low Electronegativity – low attraction of electrons • Increases as you move across a period • Decreases as you move down a group • Fluorine has highest value (4. 0)

Ionic Bond cont’ • In ionic compounds, there needs to be a large difference in electronegativity values between atoms. • Greater than 1. 7 • Metals = low electronegativity • Nonmetals = high electronegativity • Example: Na. Cl • Na = 0. 93 • Cl = 3. 16 • 3. 16 – 0. 93 = 2. 23
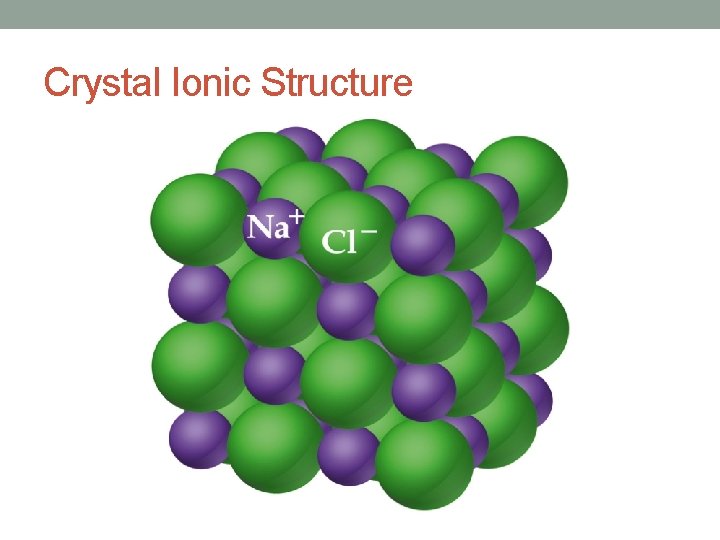
Crystal Ionic Structure

Examples of Forming an Ionic Compound • Potassium Chloride • Barium Fluoride

Metallic Bonding • Metals: • low electronegativity = Don’t attract each other’s electrons • Metals consist of closely packed cations floating in a “sea of electrons”. • All of the atoms are able to share the electrons; the electrons are not bound to individual atoms.
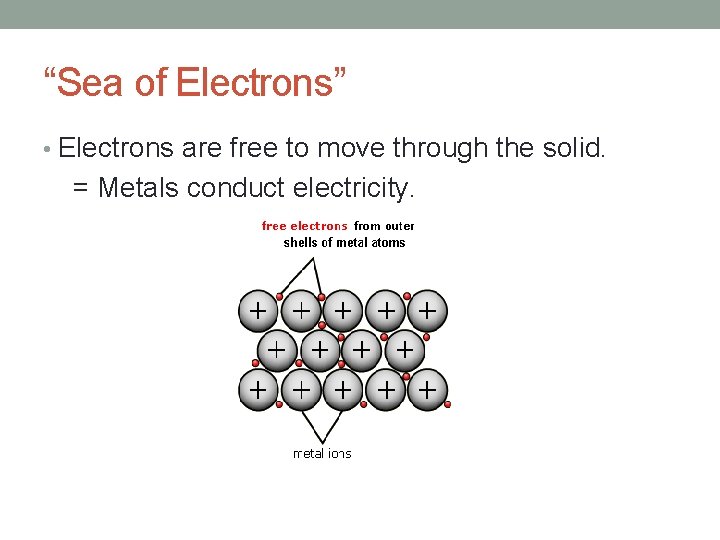
“Sea of Electrons” • Electrons are free to move through the solid. = Metals conduct electricity.
Covalent Bonding • Atoms now share pairs of valence electron. • Shared electron pair = bonding pair • Can share 1, 2 or 3 pairs of electrons to form single, double or triple bonds. • Occurs between 2 non-metals. • Example: H 2 O
Covalent Bonding • Two Types of Covalent Bonds: 1. Non-Polar Covalent Bond: • A bond formed between 2 atoms in which electrons are shared equally between the 2 atoms. • Electronegativity difference = less than 0. 5 • Example: Cl 2 • Electronegativity difference = 3. 0 -3. 0 = 0
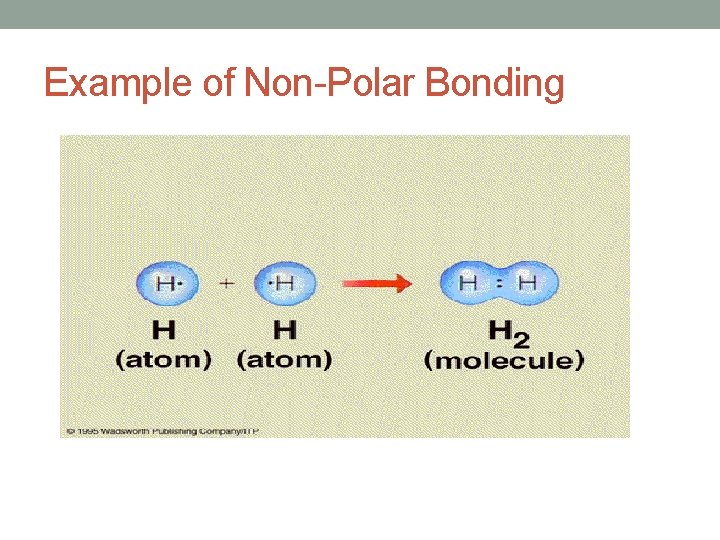
Example of Non-Polar Bonding
Covalent Bonding 2. Polar Covalent Bonding • A bond formed between 2 atoms where electrons are not equally shared. • Electronegativity difference = 0. 5 – 1. 7 • The atom with the greater electronegativity value pulls the shared electrons closer to it’s nucleus. • Example: Bond between hydrogen and oxygen • Hydrogen = 2. 1 • Oxygen = 3. 5 • Difference = 1. 4
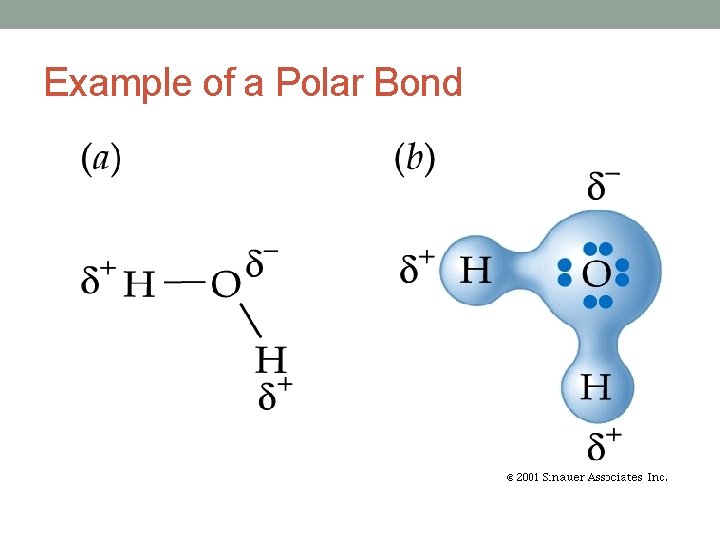
Example of a Polar Bond
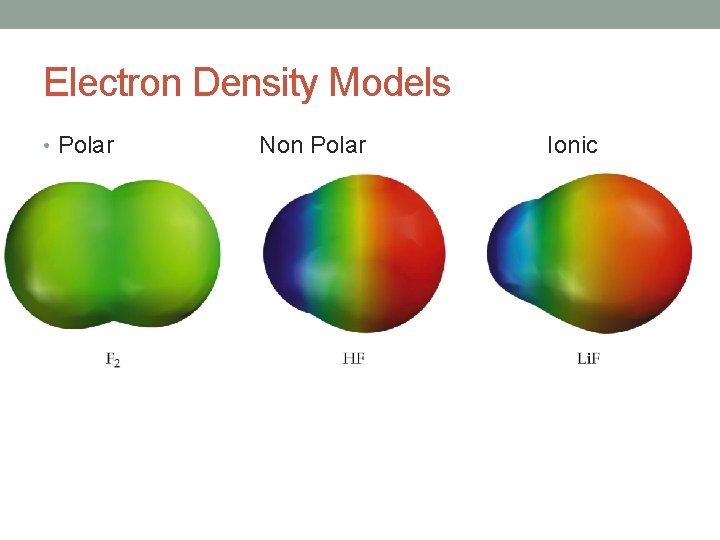
Electron Density Models • Polar Non Polar Ionic
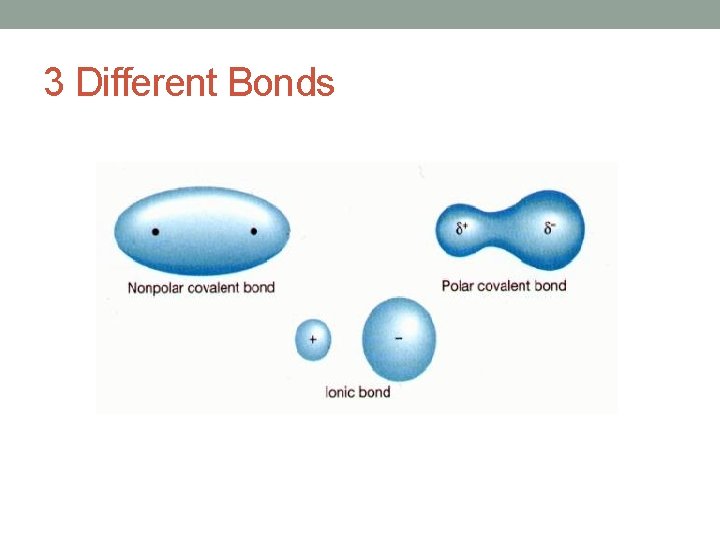
3 Different Bonds
Intramolecular vs. Intermolecular Bonding • Intramolecular Forces: • Attractive forces that occur between atoms in a molecule; chemical bonds • Examples: ionic, covalent or metallic bonds • Intermolecular Forces: • Attractive forces that occur between molecules • Examples: Dipole-Dipole attraction, hydrogen bonding, London Dispersion forces
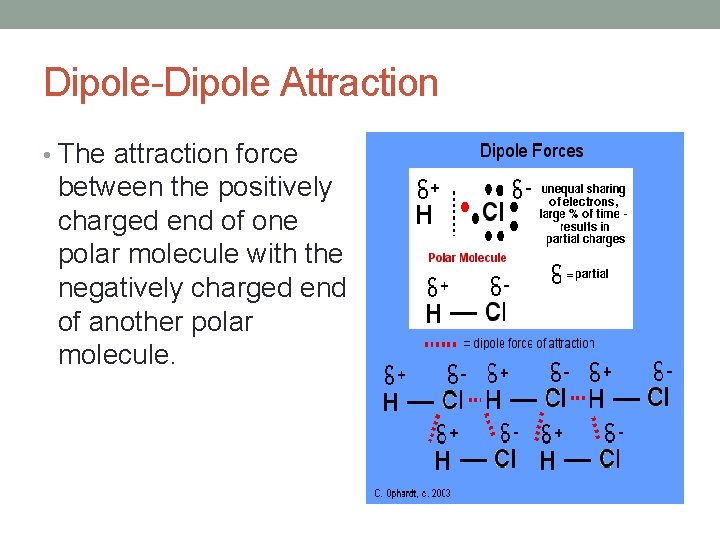
Dipole-Dipole Attraction • The attraction force between the positively charged end of one polar molecule with the negatively charged end of another polar molecule.
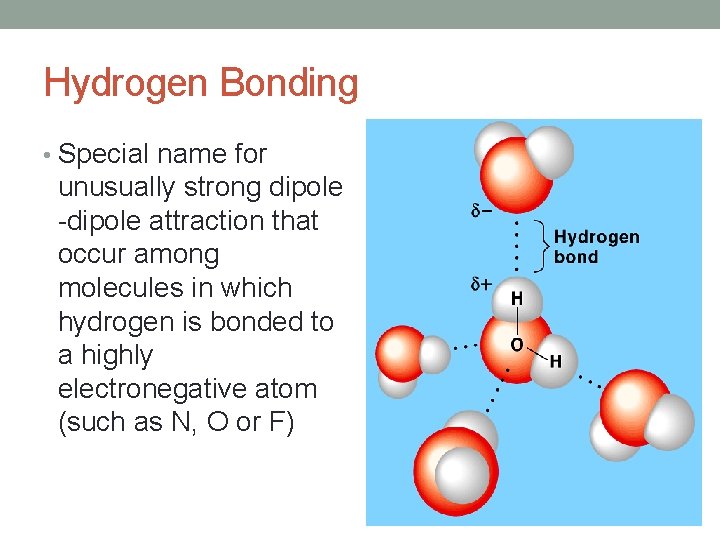
Hydrogen Bonding • Special name for unusually strong dipole -dipole attraction that occur among molecules in which hydrogen is bonded to a highly electronegative atom (such as N, O or F)

Importance of Hydrogen Bonding in Water • Responsible for water’s unique properties: • High surface tension • High melting and boiling points • High specific heat • Low density in solid form vs. liquid form • Crystal structure of ice • Insolublility in oil
- Slides: 22