Chemical Bonding UNIT 1 B MATTER QUALITATIVE ANALYSIS
Chemical Bonding UNIT 1 B - MATTER & QUALITATIVE ANALYSIS SCH 3 U/4 C

Chemical Bonding Atoms bond with each other in order to become stable. To do this, atoms will lose, gain or share valence electrons in order to obtain a stable octet.

Types of Chemical Bonds There are three types of chemical bonds that we will study in this course. Ionic bonds Pure Covalent bonds Polar Covalent bonds

Ionic Bonding In ionic bonding, electrons are completely transferred from a metal atom to a non-metal atom. This causes an electrostatic attraction between the oppositely charged ions. The bond is very strong
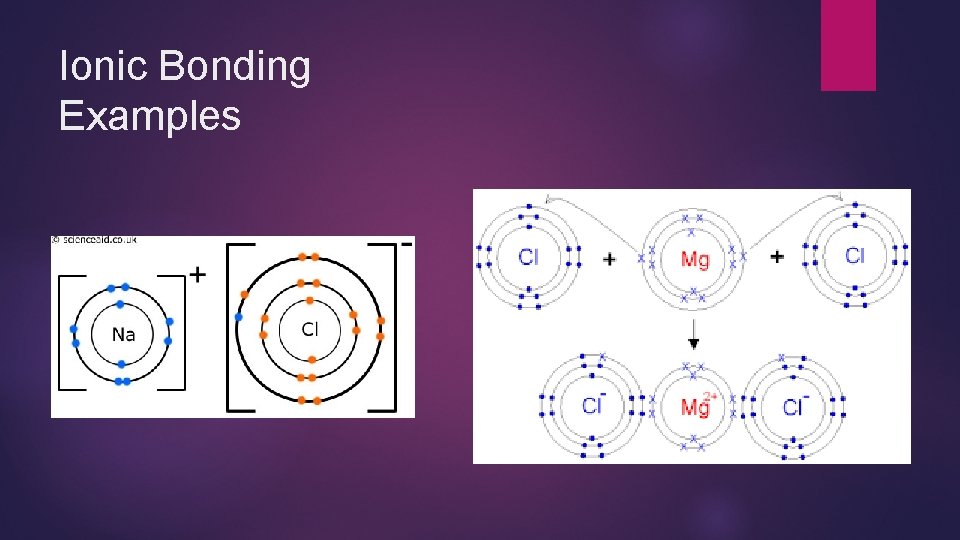
Ionic Bonding Examples
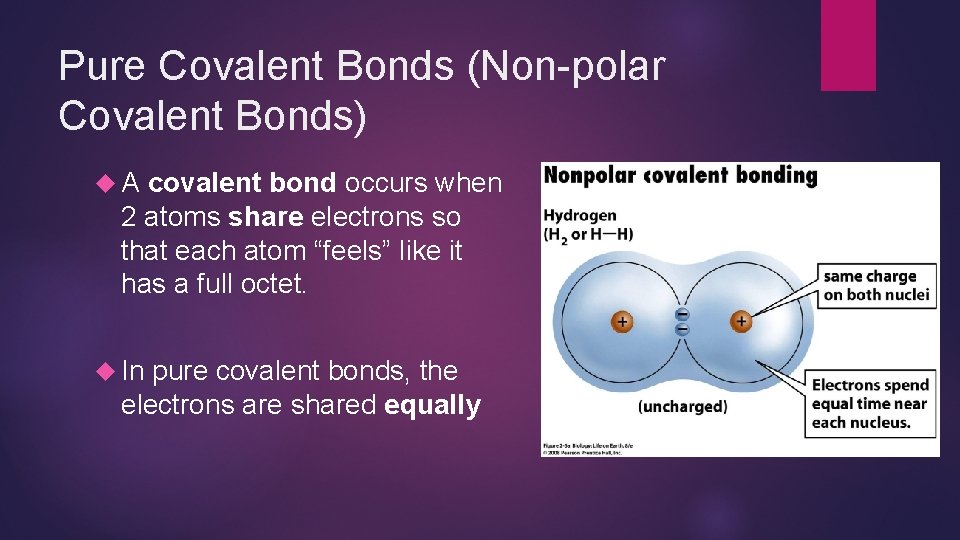
Pure Covalent Bonds (Non-polar Covalent Bonds) A covalent bond occurs when 2 atoms share electrons so that each atom “feels” like it has a full octet. In pure covalent bonds, the electrons are shared equally
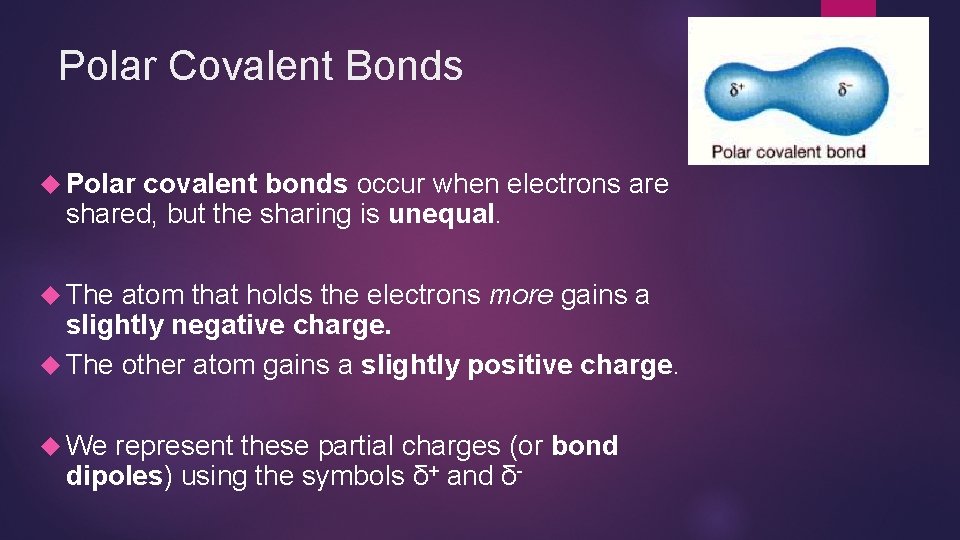
Polar Covalent Bonds Polar covalent bonds occur when electrons are shared, but the sharing is unequal. The atom that holds the electrons more gains a slightly negative charge. The other atom gains a slightly positive charge. We represent these partial charges (or bond dipoles) using the symbols δ+ and δ-
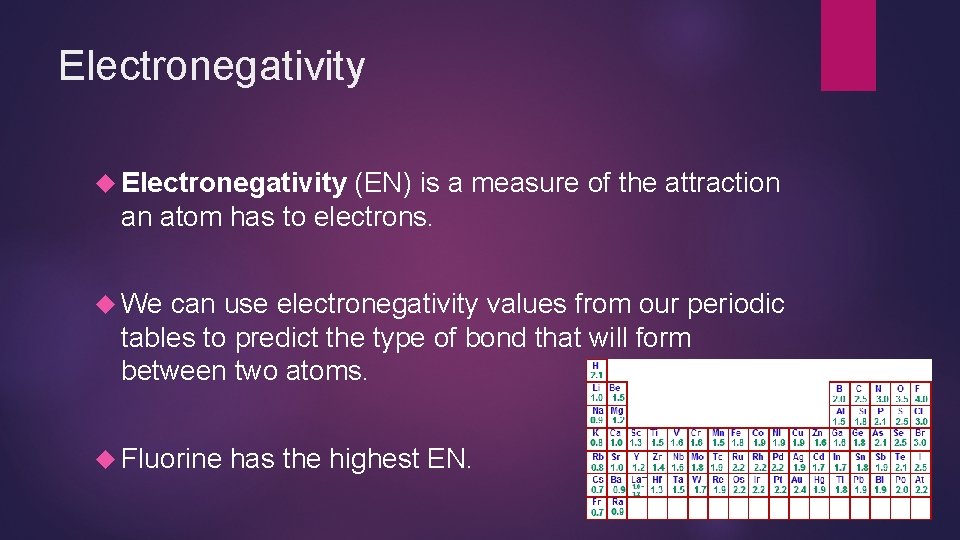
Electronegativity (EN) is a measure of the attraction an atom has to electrons. We can use electronegativity values from our periodic tables to predict the type of bond that will form between two atoms. Fluorine has the highest EN.

Predicting Bond Types To determine the type of bond formed between two atoms, identify the electronegativity values for each atom involved. Eg: Na. F EN(Na) = 0. 9 EN(F) = 4. 0

Predicting Bond Types (continued) Then determine the difference (absolute value) in electronegativities ( EN) EN = 0. 9 - 4. 0 = 3. 1 Compare this value to the bonding continuum
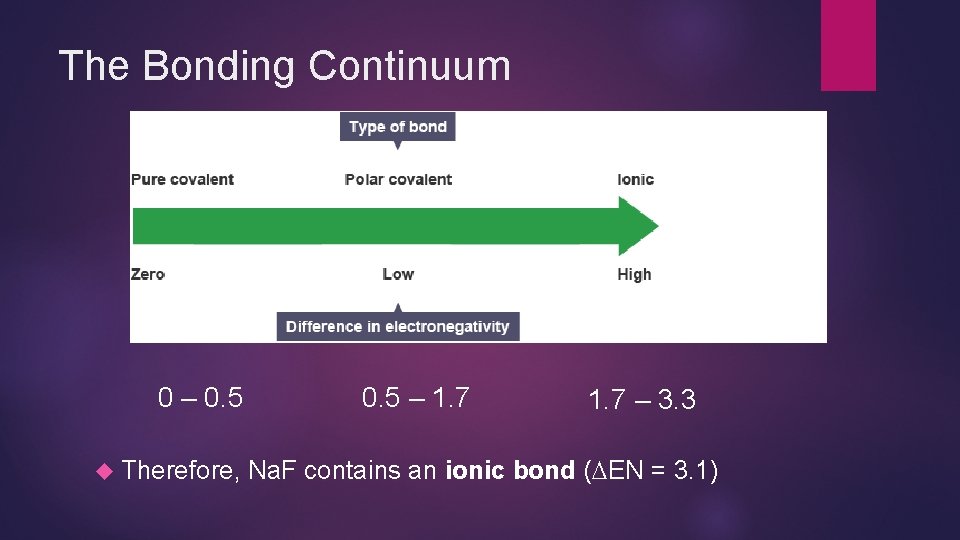
The Bonding Continuum 0 – 0. 5 Therefore, 0. 5 – 1. 7 – 3. 3 Na. F contains an ionic bond ( EN = 3. 1)
- Slides: 11